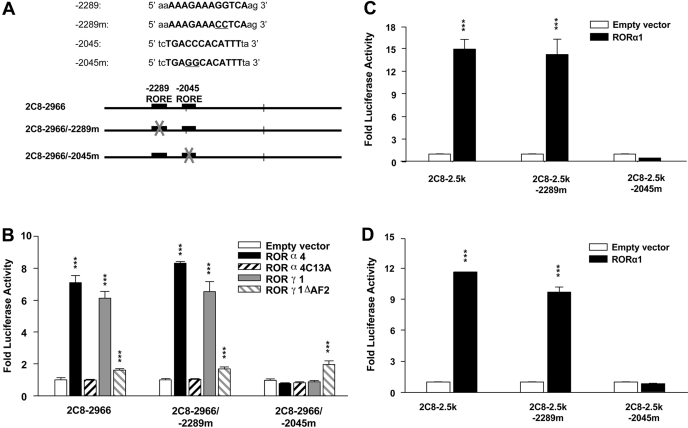
Fig. 4.
Mutational analysis of the human CYP2C8 promoter constructs to functionally characterize the two putative ROREs at -2289 and -2045 bp. A, schematic representations of the wild-type and the RORE mutated promoter constructs for transfection. B, HepG2 cells were transfected by wild-type CYP2C8-2966-bp promoter constructs or one of two CYP2C8 promoter constructs containing mutations in ROREs at -2289 or -2045, respectively. Expression plasmids of wild-type murine RORα4, its DNA binding mutant RORα4C13A, wild-type RORγ1, or a mutant lacking the AF2 domain (RORγ1ΔAF2), were cotransfected concomitantly. HepG2 (C) or Caco-2 (D) cells were similarly transfected with normal CYP2C8 promoter constructs or constructs containing mutations at either RORE and expression plasmid containing wild-type human RORα1. Twenty-four hours after transfections, cells were refreshed and maintained for another day, followed by cell lysis and luciferase activity assays. Promoter constructs were transactivated by the wild-type cotransfected RORα1, -α4, or -γ1 expression plasmids significantly compared with empty vector (***, p < 0.001) but not the RORα4 DNA binding mutant and only slightly by the RORγ mutant lacking the AF2 domain. In contrast, transactivation was abolished when the -2045RORE was mutated. Values represent the means of three independent transfections ± S.E.