Abstract
Inclusion of HIV protease inhibitors (PIs) in the treatment of people living with HIV+ has markedly decreased mortality but also increased the incidence of metabolic abnormalities, causes of which are not well understood. Here, we report that insulinopenia is exacerbated when Zucker fa/fa rats are exposed to a PI for 7 wk, suggesting that chronic PI exposure adversely affects pancreatic islet β-cell function. In support of this possibility, we find increased apoptosis, as reflected by TUNEL fluorescence analyses, and reduced insulin-secretory capacity in insulinoma cells and human pancreatic islet cells after in vitro exposures (48–96 h) to clinically relevant PIs (ritonavir, lopinavir, atazanavir, or tipranavir). Furthermore, pancreatic islets isolated from rats administered an HIV-PI for 3 wk exhibit greater cell death than islets isolated from vehicle-administered rats. The higher incidence of HIV-PI-induced cell death was associated with cleavage and, hence, activation of caspase-3 and poly(ADP)-ribose polymerase but not with activation of phospho-pancreatic endoplasmic reticulum (ER) kinase or induction of ER stress apoptotic factor C/EBP homologous protein. Exposure to the HIV-PIs, however, led to activation of mitochondria-associated caspase-9, caused a loss in mitochondrial membrane potential, and promoted the release of cytochrome c, suggesting that HIV-PIs currently in clinically use can induce β-cell apoptosis by activating the mitochondrial apoptotic pathway. These findings therefore highlight the importance of considering β-cell viability and function when assessing loss of glycemic control and the course of development of diabetes in HIV+ subjects receiving a protease inhibitor.
Keywords: human immunodeficiency virus, insulinoma cells, pancreatic islets, β-cell death, insulin secretion, cytochrome c
infection with HIV is characterized by active viral replication causing the immunodeficient state that typifies the acquired immunodeficiency syndrome (AIDS). The HIV replication cycle involves binding of the retrovirus to specific membrane receptors on target cells (i.e., CD4) and release of RNA and viral enzymes into the cytoplasm (40). To combat viral replication, a regimen of highly active antiretroviral therapy (HAART) targeted toward inhibiting the reverse transcriptase (RT) and viral protease is used. The combination therapy includes nucleoside and nonnucleoside RT inhibitors (NRTIs and NNRTIs) and HIV protease inhibitors (HIV-PIs).
Over the past two decades, the number of people worldwide living with HIV/AIDS has risen to nearly 40 million (35). The introduction of protease inhibitors in 1995 has resulted in marked decreases in mortality among HIV+ patients from 30% achieved with RT combination therapy alone to 8% with the addition of a protease inhibitor (33). The beneficial effects of protease inhibitors in combination with NRTIs/NNRTIs is evidenced by the dramatic decreases in HIV plasma viremia, in marked reductions in opportunistic infections, and in mortality and morbidity among HIV+ patients (1). However, inclusion of protease inhibitors in the therapeutic regimen is also associated with peripheral lipoatrophy, visceral adiposity, hyperlipidemia, insulin resistance, hyperglycemia, and overt type 2 diabetes mellitus (7). The development of these metabolic complications, analogous to the metabolic syndrome (37), may be affected by multiple factors, including genetic background, age, environmental/behavioral factors, anti-HIV medication exposure, host-inflammatory factors, and other medications used (12). However, they are reported to occur in 60–80% of HIV+ patients treated with protease inhibitors and are associated with significant risk for cardiovascular disorders in these patients (13).
Oral glucose tolerance and hyperinsulinemic-euglycemic clamp procedures reveal glucose intolerance and insulin resistance in HIV+ patients treated with HAART that included protease inhibitors (5, 17). Development of these metabolic abnormalities was found to be associated with the inclusion of a protease inhibitor, but less frequently associated with NRTI or NNRTI use (41) or demographic factors (33). Furthermore, the metabolic sequelae were evident in HIV-seronegative people acutely exposed to select protease inhibitors (21). In those studies, indinavir or ritonavir/lopinavir were found to impair glucose tolerance and inhibit insulin-stimulated glucose disposal. These and other findings demonstrating that several protease inhibitors cause insulin resistance and diabetes (4, 30, 42) led to the suggestion that they may be a drug class effect (24). Conversely, the recently introduced protease inhibitor atazanavir, which contains an additional ring-like structure (15), was reported to not cause insulin resistance or diabetes when administered for a short time to HIV-seronegative people (31). However, atazanavir has since been shown to disrupt the insulin-signaling pathway in cultured adipocytes (19). Thus, although atazanavir might be considered the protease inhibitor of choice, its potential metabolic effects have not been adequately elucidated.
Several mechanisms have been proposed for protease inhibitor-induced insulin resistance and diabetes. These include inhibition of glucose transporter 4 (26); decreased conversion of proinsulin to insulin (4, 18); increases in soluble type 2 TNFα receptors (28) reflecting activation of the TNF system by TNFα, a known inducer of insulin resistance (16); and reductions in the release of adipocyte-derived adiponectin, which enhances insulin-stimulated suppression of hepatic glucose production and increases in peripheral glucose disposal (27). Furthermore, “lipotoxicity” due to 1) increased subcutaneous fat loss that results in increases in circulating fatty acids (14), which when taken up by muscle inhibit insulin-signaling (11), 2) inhibition of adipogenesis leading to suppression of lipogenesis and stimulation of lipolysis (23), 3) dysregulation of CD36 (9), a facilitator of fatty acid uptake, or iv) decreases in adipocyte-derived perilipin, a regulator of lipid metabolism, leading to stimulation of lipolysis (39) is thought to act in concert with other metabolic perturbations to promote protease inhibitor-induced insulin resistance and diabetes.
Collectively, these studies suggest that the manifestation of insulin resistance and diabetes during protease inhibitor treatment is associated with dysregulation of various factors and pathways. Unfortunately, a limitation of these studies is that they involved acute exposures to protease inhibitors under in vitro conditions. Furthermore, very few studies have considered the potential effects of protease inhibitors on pancreatic β-cells. In view of the overwhelming evidence that the beneficial effects of protease inhibitors must be balanced against their potential to induce metabolic abnormalities, it is important to gain a better understanding of the mechanism(s) by which protease inhibitors induce these effects so that improved drugs and therapeutic regimens can be developed. Here, we present evidence for decreases in viability and secretory function of β-cells exposed to clinically used HIV-protease inhibitors under in vitro and in vivo conditions.
RESEARCH DESIGN AND METHODS
Materials.
The HIV-protease inhibitors were supplied by the pharmacy at the Washington University School of Medicine AIDS Clinical Trials Unit (St. Louis, MO) or The AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, and NIH (http://www.aidsreagent.org/). Zucker wild-type lean (ZWT) and Zucker diabetic fatty (ZDF) rats were purchased from Charles River Laboratories (Wilmington, MA). 832/13 INS-1 insulinoma cells were generously provided by Dr. C. Newgard (Duke University Medical Center, Durham, NC). Human islets were obtained through the ICR Basic Science Islet Distribution Program. Other materials were obtained from the following (sources): rainbow molecular mass standards and enhanced chemiluminescence reagent (Amersham, Arlington Heights, IL); SYBR Green PCR Kit (Applied Biosystems, Foster City, CA); Coomassie reagent, SDS-PAGE supplies, and Triton X-100 (Bio-Rad, Hercules, CA); Hanks' balanced salt solution (HBSS), heat-inactivated fetal bovine serum, l-glutamine, and CMRL tissue culture medium (GIBCO, Grand Island, NY); Immobilon-P PVDF membrane (Millipore, Bedford, MA); in situ cell death detection (Roche Diagnostics, Indianapolis, IN); primary and secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); common reagents, protease inhibitor cocktail (PIC), and salts (Sigma Chemical, St. Louis, MO); and penicillin and streptomycin (Tissue Culture Center, Washington University, St. Louis, MO).
ZDF and ZWT rats.
Five-week-old male ZDF and ZWT rats were maintained in a 12:12-h light-dark cycle, weighed daily, and fed commercial rat chow (Ralston-Purina, St. Louis, MO.) with free access to water. Food consumption was measured 4 days a week, and rats were pair fed to equalize food consumption among all groups. All animal procedures were approved by the Animal Studies Committee at Washington University School of Medicine (St. Louis, MO).
Vehicle and indinavir administration to rats.
Five-week-old ZDF rats (n = 8 in each group) were randomly separated into eight groups and administered either vehicle, two nucleoside reverse transcriptase inhibitors (NRTIs), indinavir (IDV, 170 mg/kg), or NRTIs + IDV. The NRTI combinations used were didanosine (ddl, 29 mg/kg) + lamivudine (3TC, 43 mg/kg); stavudine (d4T, 5.5 mg/kg) + 3TC; or zidovudine (AZT, 21 mg/kg) + 3TC. The drugs were suspended in vehicle (sterile water) and administered by oral gavage twice a day for 7 wk to mimic the route of administration in humans. The ZWT rats were separated into two groups and treated with either vehicle or IDV alone. The IDV dose and route of administration were based on rodent studies (8, 10) and the high metabolic rate of rodents. At weekly intervals starting at 5 wk of age, an aliquot of blood was obtained by tail snip from overnight-fasted rats. Plasma glucose concentration was determined using an automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH), and plasma insulin concentration was determined using an ultrasensitive rat insulin ELISA kit (Crystal Chem, Downers Grove, IL), according to the manufacturer's instructions.
INS-1 cell culturing and treatment.
The 832/13 INS-1 cells were seeded in 24-well plates and cultured in RPMI 1640 medium containing 10% fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, 1 mM Na-pyruvate, 50 μM β-mercaptoethanol, 11 mM glucose, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C and 5% CO2-95% air, as described (36). At ∼75% confluence, the culturing medium was replaced with medium containing DMSO alone or with 20 μM indinavir, ritonavir, lopinavir, atazanavir, or tipranavir. The concentration of protease inhibitor chosen was based on earlier acute studies examining glucose uptake (20). The drug-containing medium was replaced every 12 h with fresh medium containing DMSO or protease inhibitors. Insulin content in the medium was determined over a 48-h period by RIA. The cells were then harvested to determine by flow cytometry analyses the incidence of apoptosis. Additionally, following exposure to 1–20 μM ritonavir (RTV) or atazanavir (ATV) for 48 h, the cells were equilibrated in medium containing 0 mM glucose for 1 h. The medium was then replaced with one containing either 1 mM glucose or 20 mM glucose. After 1 h, insulin content in the medium was measured by RIA, and INS-1 cell protein concentration was determined using Coomasie reagent.
Human islet culturing and treatment.
Human islet suspensions provided by the ICR were immediately cleaned of nonislet material, and 500 islets/2 ml complete CMRL 1066 (cCMRL, containing 10% fetal bovine serum, 100 U/ml penicillin, 2 mM glutamine, and 25 mM HEPES, 5.5 mM glucose, pH 7.4) were cultured overnight at 37°C under an atmosphere of 5% CO2-95% air. Each batch of islets was then incubated with DMSO vehicle or 20 μM indinavir (IDV), RTV, or ATV. The medium was changed every 12 h for up to 96 h. The islets were washed twice with Krebs-Ringer buffer [KRB, containing (in mM): NaCl (115), NaHCO3 (24), KCl (5), MgCl2 (1), HEPES (25), glucose (1), and 0.10% BSA, pH 7.3] and divided into groups of 20 islets. The islets were equilibrated for 1 h in KRB, and the buffer was then replaced with one containing either 3 mM glucose or 20 mM glucose. After 1 h, insulin content in the medium was measured by RIA and islet protein concentration using Coomasie reagent.
Rat islet isolation.
Islets were isolated from ZWT rats administered vehicle or IDV for 3 wk after pancreatic excision, collagenase digestion, centrifugation through a discontinuous Ficoll gradient, and manual selection under stereomicroscopic visualization to exclude contaminating tissues, as described (25). The islets were resuspended in cCMRL, transferred into Falcon Petri dishes containing 2.5 ml of cCMRL, and cultured overnight under an atmosphere of 5% CO2-95% air at 37°C. The islets were then harvested and mounted onto slides by cytospin and processed for TUNEL analyses, as described below.
TUNEL staining to detect DNA cleavage and apoptosis.
TUNEL staining analyses were done using a kit, according to manufacturer's instructions. INS-1 cells exposed to vehicle or protease inhibitor were harvested at 24 and 48 h and washed twice with ice-cold phosphate-buffered saline (PBS). INS-1 cell apoptosis was assessed by measuring TUNEL fluorescence by flow cytometry, and the percentage of TUNEL-positive cells was determined by analyzing 10,000 cells.
Islets (human or rat) were immobilized on slides by cytospin and fixed with 4% paraformaldehyde (in PBS, pH 7.4, 1 h, room temperature). The islets were then washed (3 × 5 min) with PBS and incubated in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate in PBS, 30 min, room temperature). The permeabilization solution was then removed, TUNEL reaction mixture (50 μl) was added, and the islets were incubated in a humidified chamber (1 h, 37°C). The islets were washed again with PBS (3 × 5 min) and counterstained with 1 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) in PBS for 10 min to identify cellular nuclei. Incidence of islet cell apoptosis was assessed under a fluorescence microscope using an FITC filter. Islet cells with TUNEL-positive nuclei were considered apoptotic. DAPI staining of nuclei was used to identify islet cell number, and the percentage of TUNEL-positive cells relative to total cell number was determined.
Assessment of INS-1 cell mitochondrial membrane potential by flow cytometry.
Loss of mitochondrial membrane potential (MitoMP) is an important step in the induction of cellular apoptosis. INS-1 cell MitoMP was measured using a commercial kit according to the manufacturer's instructions, as described (22). Briefly, harvested cells were washed once with PBS and resuspended in 100 μl of the same buffer (∼105 cells/ml). Mito Flow fluorescent reagent (5 μl) was added, and the cell suspension was incubated at 37°C for 30 min. The cells were then transferred to fluorescence-activated cell sorting tubes and diluted 1:5 with buffer provided in the kit. Fluorescence in cells was analyzed by flow cytometry (BD Biosciences, San Jose, CA) at an excitation wavelength of 488 nm.
Subcellular fraction preparation.
INS-1 cells were harvested and washed twice (750 g, 5 min, 4°C) with 10 volumes of ice-cold PBS. The cell pellet was suspended in 3 volumes of ice-cold isolation buffer (20 mM HEPES-KOH, pH 7.8, 250 mM sucrose, 1 mM EGTA, 10 mM potassium chloride, supplemented with protease inhibitor cocktail (PIC; 50 μl/ml). The cells were placed on ice for 15 min and then transferred to a Dounce homogenizer (Kimble/Kontes, Vineland, NJ) and disrupted by douncing 15 times on ice. The homogenate was centrifuged at 800 g for 5 min to remove unbroken cells and nuclei, and the supernatant was centrifuged at 15,000 g for 15 min at 4°C to obtain mitochondria. The supernatant was subjected to further ultracentrifugation at 100,000 g. The resultant supernatant was the cytosolic fraction.
Immunoblotting analyses of apoptotic factors.
An aliquot (containing 30 μg of protein) of cytosol was analyzed by SDS-PAGE (8 or 15%), transferred onto Immobilin-P PVDF membranes, and processed for immunoblotting analyses, as described (22). The targeted proteins and the ([1° antibody]) were as follows: phosphorylated phospho-pancreatic endoplasmic reticulum (ER) kinase (PERK; 1:1,000), C/EBP homologous protein (CHOP; 1:500), caspases (1:1,000), poly(ADP)-ribose polymerase (PARP; 1:1,000), cytochrome c (1:1,000), and tubulin (1:500) or GAPDH (1:200) control. Secondary antibody dilutions for PARP and tubulin were 1:5,000 and for the rest were 1:10,000. Immunoreactive bands were visualized by enhanced chemiluminescence.
RESULTS
Long-term administration of IDV to rats promotes a decrease in circulating insulin levels.
Inclusion of protease inhibitors in the treatment of people living with HIV+ is recognized to be associated with metabolic complications that includes onset of type 2 diabetes mellitus (T2DM). Because most studies to date have examined the development of insulin resistance and hyperglycemia following protease inhibitor exposures under acute and in vitro conditions, we examined the effects of long-term (7 wk) and in vivo IDV exposures on circulating glucose and insulin levels in ZDF rats that are genetically predisposed to developing T2DM. We found that administration of IDV to ZDF rats increases circulating glucose levels within 3 wk, relative to vehicle-administered rats, and that this occurs despite a parallel rise in insulin levels during the first 2–4 wk (Fig. 1, A and B). Surprisingly, although the hyperglycemic state worsened during the final 3 wk of IDV exposure, insulin levels progressively decreased during this period. As illustrated in Fig. 2, A and B, the average glucose levels were higher and the insulin levels lower in the IDV group during weeks 5–7 of administration compared with the vehicle-administered group.
Fig. 1.
Weekly fasting plasma glucose and insulin levels in Zucker diabetic fatty (ZDF) rats administered nucleoside RT inhibitors (NRTIs) ± indinavir (IDV). Male ZDF rats were administered vehicle, IDV, or NRTIs ± IDV from 5 to 12 wk of age. An aliquot of blood was collected at weekly intervals from overnight-fasted rats for plasma glucose and insulin measurements, and weekly mean values ± SE (n = 8 in each group) are presented. A: plasma glucose. B: plasma insulin levels. IDV (170 mg/kg), ddl (didanosine, 29 mg/kg), 3TC (lamivudine, 43 mg/kg), d4T (stavudine, 5.5 mg/kg), and AZT (zidovudine, 21 mg/kg) were administered to the rats, as described in research design and methods.
Fig. 2.
Average fasting plasma glucose and insulin levels in ZDF rats during final 3 wk of NRTIs ± IDV administration. Male ZDF rats were administered vehicle, IDV, or NRTIs ± IDV from 5 to 12 wk of age, as described in Fig. 1. An aliquot of blood was collected at weekly intervals from overnight-fasted rats for plasma glucose and insulin measurements, and average mean values ± SE (n = 8 in each group) during weeks 5–7 of administration are presented. A: plasma glucose. *Significantly different from placebo group, P < 0.0001; #,†IDV groups significantly different from corresponding −IDV groups, where P < 0.01 and P < 0.0001, respectively; §significantly different from IDV-alone group. B: plasma insulin levels. #,†IDV groups significantly different from corresponding −IDV groups, where P < 0.05 and P < 0.005, respectively.
In comparison, NRTI combination regimens [didanosine (ddl) + lamivudine (3TC), stavudine (d4T) + 3TC, and AZT + 3TC)] induced a delayed rise in glucose levels starting at 5 wk (Fig. 1A) that was not accompanied by a decrease in insulin levels during the final 3 wk of administration (Fig. 1B). In fact, average circulating insulin levels during the last 3 wk in NRTI-administered rats were similar to those in vehicle-administered animals (Fig. 2B). However, coadministration of IDV with the NRTIs accelerated the onset of a rise in glucose levels (Fig. 1A) and exacerbated the hyperglycemia during the final 3 wk of administration (Fig. 2A) that was now accompanied by decreases in insulin levels (Fig. 2B), similar to the IDV-alone-administered group. These findings suggest that, in addition to promoting an insulin resistance state, protease inhibitors may also have previously unrecognized effects on β-cell viability and/or secretory capacity. We therefore addressed this possibility in the absence of background hyperglycemia or hyperlipidemia in studies described below using insulinoma cells, isolated human pancreatic islets, and rat pancreatic islets isolated from ZWT rats.
IDV exposure leads to apoptosis of insulinoma cells.
To assess the effects of protease inhibitors on β-cell viability, 832/13 INS-1 cells, a genetically engineered insulinoma cell line that exhibits higher insulin-secretory capacity than parental cells and is widely used to study β-cell function, were exposed to IDV for up to 48 h. The cells were harvested at various times, and flow cytometry was used to quantitate TUNEL-positive cells. This assay relies on the gain in fluorescence of cells undergoing apoptosis, which causes a shift in the fluorescence intensity peaks to the right. Although there were minimal changes at 24 h (data not shown), exposure to IDV for 48 h caused a significant shift in the fluorescence peak to the right of the control peak (Fig. 3A), suggesting an increase in the population of cells undergoing apoptosis. Quantitation of TUNEL-positive cells revealed a twofold increase in the percentage of apoptotic cells following IDV exposure (Fig. 3B). These findings are the first demonstration of HIV-protease inhibitor-induced β-cell apoptosis.
Fig. 3.
IDV-induced insulinoma cell apoptosis. 832/13 INS-1 cells were exposed to vehicle (V) or 20 μM IDV for 48 h and then harvested and processed for TUNEL analyses by flow cytometry. Representative spectra of protease inhibitor (PI)-induced rightward shift in fluorescence, indicative of an increase in TUNEL-positive cells, are presented. A: TUNEL flow cytometry spectra. B: TUNEL-positive cell number. Data are presented as mean ± SE of the percentage of apoptotic cells in each group (n = 4–11 in each group). *IDV-administered group significantly different from vehicle, where P < 0.001.
Effects of HIV-protease inhibitors in clinical use on INS-1 cell viability.
On the basis of the results with IDV and because previous studies utilized 20 μM concentration of various HIV-protease inhibitors to examine insulin-secretory function (20), we examined whether a similar concentration of ritonavir (RTV), lopinavir (LPV), and the newer-generation protease inhibitors ATV and tipranavir (TPV) caused INS-1 cell apoptosis after a 48-h exposure period. As shown in Fig. 4A, these protease inhibitors also induced a rightward-shift in TUNEL fluorescence that was reflected by a two- to threefold increase in the percentage of apoptotic cells (Fig. 4B). The fold increase in apoptosis induced by each protease inhibitor, relative to vehicle, was as follows: LPV (3.3 ± 0.2) > RTV (2.5 ± 0.2) = ATV (2.7 ± 0.3) > TPV (1.6 ± 0.3). These findings indicate that HIV protease inhibitors in clinical use can cause apoptosis of insulinoma cells.
Fig. 4.
Effects of HIV-PIs in clinical use on INS-1 cell viability. A: TUNEL flow cytometry. INS-1 cells were exposed to vehicle (V), ritonavir (RTV), lopinavir (LPV), atazanavir (ATV), or tipranavir (TPV) for 48 h and then harvested and processed for TUNEL analyses as in Fig. 3. B: TUNEL-positive cell number. TUNEL-positive (apoptotic) cells in each group were quantitated, and data are presented as mean ± SE of the percentage of apoptotic cells in each group (n = 4–8 in each group). *,#PI-administered group significantly different from corresponding vehicle, where P < 0.001 and P < 0.01, respectively.
HIV protease inhibitors decrease insulin secretion from insulinoma cells.
To determine whether the higher incidence of apoptosis associated with the protease inhibitors is reflected by changes in insulin-secretory capacity, basal and glucose-stimulated insulin secretion from the INS-1 cells were determined. As shown in Fig. 5A, basal insulin secretion was reduced following exposure to TPV for 3 h. At 24 h, insulin secretion was decreased by all protease inhibitors except ATV. However, following exposure for 48 h, basal insulin secretion was decreased in all groups. Furthermore, glucose-stimulated insulin secretion at this time was decreased from cells exposed to 1–20 μM RTV or ATV relative to secretion from vehicle-exposed cells (Fig. 5B). The findings with the insulinoma cells therefore reveal for the first time that protease inhibitors may have direct effects on the viability and secretory function of these cells and suggest that the HIV protease inhibitors in clinical use could have adverse affects on pancreatic islet β-cell viability and function. They further suggest that longer exposures to even the newer-generation HIV protease inhibitors (ATV and TPV) can induce decreases in cell viability and function.
Fig. 5.
HIV-PI-induced decrease in basal and glucose-stimulated insulin secretion from insulinoma cells. A: basal insulin secretion. INS-1 cells were exposed to PIs as in Fig. 4, and insulin content in a 50-μl aliquot of medium collected at various times during 48-h exposure period was determined by RIA. Data are mean ± SE percentage values of insulin secretion from PI-exposed cells relative to cells exposed to only vehicle (n = 6–14 in each group). *,#PI-administered group significantly different from vehicle group, where P < 0.05 and P < 0.005, respectively. B: glucose-stimulated insulin secretion. After 48-h exposure period to 1–20 μM ritonavir (R) or atazanavir (A), cells were incubated in medium containing 1 or 20 mM glucose for 1 h. Insulin content in the medium was then determined by RIA. *,#Significantly different from control 20G responses, P < 0.005 and P < 0.01, respectively.
HIV protease inhibitors induce apoptosis of human pancreatic islet cells.
To examine the effects of HIV protease inhibitors on islet cells, we utilized native human pancreatic islets, which were prepared and provided to us by an islet procurement site (through the ICR and JDRF) site within 24 h of isolation. Due to the limited availability of the human islets, we compared one older- (RTV) with one newer-generation (ATV) protease inhibitor. The islets were exposed to vehicle, 1–20 μM RTV, or 1–20 μM ATV for up to 96 h, and the incidence of islet cell apoptosis was assessed by TUNEL staining analyses using fluorescence microscopy. The percentages of apoptotic cells relative to total cell number, determined by counting DAPI-stained cell nuclei, were found to be significantly higher in the RTV and ATV groups than in vehicle-exposed islets (Fig. 6A). Whereas concentration- and time-dependent increases in apoptosis were seen with RTV, the apoptosis induced by ATV was found to be similarly increased at all concentrations and times tested (Fig. 6A). Moreover, islets exposed to the protease inhibitors tended to be smaller than islets exposed to vehicle only (Fig. 6B). Although these analyses are more likely to identify surface islet cells, these findings suggest that pancreatic islet cells are susceptible to undergoing apoptosis following exposure to HIV protease inhibitors that are currently in clinical use.
Fig. 6.

HIV-PI-induced apoptosis of human pancreatic islet cells. A: TUNEL-positive cell quantitation. Human islets were exposed to vehicle, RTV (1–20 μM), or ATV (1–20 μM). TUNEL analyses were done at 24, 48, 72, and 96 h, and the mean ± SE of the percentage of apoptotic cells, relative to total number of DAPI-stained cells in each group (n = 7–8 in each group) are presented. *,#PI-administered group significantly different from vehicle, P < 0.05 and P < 0.005, respectively. B: DAPI and TUNEL staining of islet cells. Islets were processed for TUNEL analyses as described in research design and methods. DAPI stain was used to identify cell nuclei and TUNEL stain to identify apoptotic cells. Brightly fluorescing cells represent TUNEL-positive or apoptotic cells within the same islet.
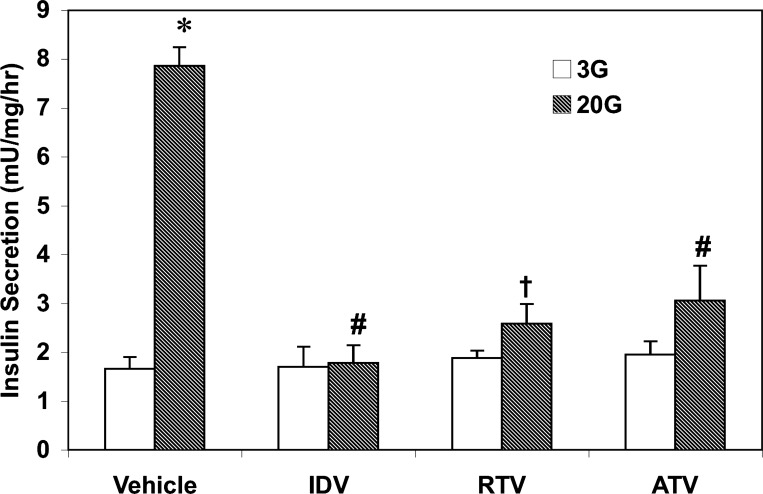
HIV protease inhibitors decrease glucose-stimulated insulin secretion from human pancreatic islets.
We next examined whether protease inhibitor-induced apoptosis of human pancreatic islet cells affects stimulated insulin secretion from these islets. Human pancreatic islets were exposed to vehicle or protease inhibitors for 96 h and subsequently treated with either 3 mM glucose or 20 mM glucose. As shown in Fig. 7, insulin secretion from vehicle-treated islets in the presence of 20 mM glucose was nearly fivefold higher than under basal conditions (3 mM glucose). In contrast, although basal secretion was unaffected by exposure to IDV, RTV, or ATV, insulin secretion from islets exposed to the protease inhibitors in response to 20 mM glucose was decreased by 60–80%. Collectively, the findings in human islets indicate that HIV protease inhibitors in clinical use affect native pancreatic islet β-cell viability and insulin-secretory capacity and suggest that their in vivo effects on pancreatic islet cell viability and function need to be considered.
Fig. 7.
HIV-PI-induced decrease in glucose-stimulated insulin secretion from human pancreatic islets. Human pancreatic islets were first exposed to vehicle, RTV, or ATV for 96 h. Islets from each group were then separated into 20 islet batches and incubated in KRB containing 3 or 20 mM glucose for 1 h. Insulin release in a 50-μl aliquot of medium was then determined by RIA. Data are mean ± SE percentage values of insulin secretion from PI-exposed islets relative to islets exposed to only vehicle (n = 4–6 in each group). *Vehicle 20G group significantly different from vehicle 3G group, P < 0.0001; #,†significantly different from vehicle 20G group, P < 0.001 and P < 0.005, respectively.
Long-term treatment of ZWT rats with IDV induces pancreatic islet cell apoptosis.
Because data obtained in ZDF rats (Figs. 1 and 2) suggested a decrease in insulin secretion with IDV exposures, we examined the in vivo effects of IDV administration on pancreatic islets in ZWT lean rats, which are not predisposed to becoming diabetic or hyperlipidemic. The rats were administered either vehicle or IDV for 3 wk, and pancreatic islets were isolated and processed for TUNEL analyses. Unexpectedly, the number of islets recovered from the IDV-administered rats was nearly 30–50% of the islet yield from vehicle-administered ZWT rats, and this precluded the possibility of performing insulin secretion assays or continuing IDV administration for a longer time. Furthermore, whereas vehicle-exposed islets appeared to maintain uniform structure and had few TUNEL-positive cells (Fig. 8A), IDV-exposed islets lost their shape and contained nearly fourfold greater number of TUNEL-positive cells (Fig. 8B).
Fig. 8.
Induction of islet cell apoptosis in Zucker wild-type (ZWT) rats administered IDV for 3 wk. Male ZWT lean rats were administered either vehicle (V) or IDV (170 mg/kg twice a day) from 3 to 6 wk of age, as in Fig. 1. A: TUNEL analyses. Islets were isolated from 3-wk vehicle- and IDV-administered rats and processed for TUNEL analyses, as in Fig. 5. B: TUNEL-positive cell quantitation. Data are presented as mean ± SE of the percentage of apoptotic cells relative to total number of DAPI-stained cells in each group (n = 11 in each group). *IDV-administered group significantly different from vehicle, P < 0.001.
HIV protease inhibitors do not induce ER stress factors in INS-1 cells.
To elucidate a mechanism by which protease inhibitors induce β-cell apoptosis, we examined whether they do so by causing ER stress, as suggested in a recent study (34). ER stress response is associated with phosphorylation of PERK leading to its activation and increased expression of the ER apoptotic factor CHOP. However, as shown in Fig. 9, there was neither a change in the abundance of activated PERK in pancreatic islets isolated from rats administered IDV for 3 wk (Fig. 9A), nor was there a significant increase in CHOP immunoreactivity in INS-1 cells exposed to protease inhibitors for 48 h (Fig. 9B). These findings suggest that HIV protease inhibitor-induced β-cell apoptosis occurs, most likely, via an ER stress-independent pathway.
Fig. 9.
Immunoblotting analyses of endoplasmic reticulum (ER) factors following HIV-PI exposure. A: phosphorylated phospho-pancreatic ER kinase (PERK) immunoreactivity in pancreatic islets. Pancreatic islets were isolated from rats administered either vehicle or indinavir (n = 3 in each group) for 3 wk, and expression of ER stress factor phosphorylated PERK was examined. B: C/EBP homologous protein (CHOP) immunoreactivity in INS-1 cells. INS-1 cells were exposed to vehicle or PIs for 48 h, and expression of ER stress apoptotic factor CHOP was examined.
Activation of caspases following exposure to HIV protease inhibitors.
Activation of caspases, through their cleavage, is required for the execution of apoptosis. As shown in Fig. 10, HIV protease inhibitors promoted generation of active cleaved caspases, including mitochondria-associated caspase-9. A key protein that is selectively cleaved at the onset of apoptosis by caspases is poly(ADP-ribose) polymerase (PARP) (29). Cleavage of PARP leads to generation of an active product that facilitates cellular disassembly (32), and exposure of INS-1 cells to protease inhibitors for 48 h resulted in the cleavage of PARP to the 89-kDa active product, supporting activation of caspases and induction of apoptosis by the protease inhibitors. These findings raised the possibility that β-cell apoptosis due to HIV protease inhibitors occurs via the mitochondrial apoptotic pathway.
Fig. 10.
Activation of caspases and poly(ADP)-ribose polymerase (PARP) following HIV-PI exposure. INS-1 cells were exposed to vehicle or PIs for 48 h and activation (i.e., cleavage) of caspase-8, -9, and -3 and of PARP were examined by immunoblotting analyses. Tubulin was used as loading control.
HIV protease inhibitors promote MitoMP loss and cytochrome c release.
To address the possibility that HIV protease inhibitors induce the mitochondrial apoptotic pathway, we monitored MitoMP and cytosolic cytochrome c accumulation following exposure to these drugs. Loss of MitoMP is a hallmark of cellular apoptosis, and this was assessed in a suspension of cells to which a fluorescent Mito Flow reagent was added. The reagent concentrates in the mitochondria of healthy cells, but mitochondria of cells undergoing apoptosis become compromised and accumulate less of the reagent, and this is reflected by a decrease in the fluorescence signal and emergence of a second peak to the left of the original. This peak represents cells losing MitoMP and their percentage, relative to total cell number, was analyzed by the application software and is indicated as M1. The spectra presented in Fig. 11A reflect fluorescence measurement in 10,000 INS-1 cells following exposure to vehicle, RTV, or ATV for 24 and 48 h. As illustrated in Fig. 11, A and B, RTV at 24 and 48 h and ATV at 48 h induced losses in MitoMP relative to the vehicle group, and this was accompanied by increased accumulation of cytochrome c in the cytosol (Fig. 11C). Both of these events are recognized to occur during activation of the mitochondrial apoptotic processes, suggesting that β-cell apoptosis due to HIV protease inhibitors most likely involves the intrinsic pathway.
Fig. 11.
HIV-PIs induce loss of mitochondrial membrane potential (MitoMP) and cytochrome c release. INS-1 cells were exposed to DMSO vehicle, 20 μM RTV, or 20 μM ATV. Cells were harvested at 24 or 48 h for MitoMP measurement by flow cytometry and immunoblotting analyses of cytochrome c in the cytosol. A: MitoMP. Spectra illustrate mitochondrial marker fluorescence measured in 10,000 cells. Left peak in each spectrum is a reflection of the cell population in which MitoMP is compromised, resulting in a decrease in mitochondrial marker fluorescence. B: decompensated INS-1 cells. Data represent mean ± SE values (n = 4 in each group) of the percentage of INS-1 cells with compromised MitoMP. *,#,†PI group significantly different from DMSO group, where P < 0.01, P < 0.005, and P < 0.0001, respectively. C: cytochrome c immunoreactivity. Cytosol was prepared from INS-1 cells as described in research design and methods and processed for immunoblot analyses. Cytochrome c and GAPDH loading control immunoreactivity in the same samples are presented.
DISCUSSION
Although protease inhibitor use has significantly reduced the incidence of morbidity and mortality among people living with HIV+, a number of metabolic complications are reported to occur with their use. They include development of peripheral lipoatrophy, visceral adiposity, hyperlipidemia, insulin resistance, hyperglycemia, and overt T2DM (7). Recently, we demonstrated a dramatic exacerbation of hyperglycemia and glucose intolerance in male ZDF rats, genetically predisposed to developing diabetes, administered a HIV protease inhibitor for 7 wk (6). These findings are consistent with development of an abnormal metabolic profile that is associated with HIV protease inhibitors.
Here, we report that continued administration of IDV to the ZDF rats causes a decrease in circulating insulin levels. In contrast, NRTIs, while also exacerbating hyperglycemia in the ZDF rats, did not cause a decrease in insulin levels. However, coadministration of a protease inhibitor with the NRTIs resulted in a decrease in insulin levels that was similar to that seen with the protease inhibitor alone. These findings reaffirm that protease inhibitors included in the treatment regimen contribute significantly to the increased incidence of metabolic abnormalities observed in people living with HIV+. They further raise the possibility that protease inhibitors can interfere with the normal functioning of pancreatic islet β-cells.
In the present study, we examined this possibility using insulinoma cells, human pancreatic islets, and rat pancreatic islets following short in vitro and longer in vivo exposures to protease inhibitors. We compared the effects of a first-generation HIV protease inhibitor, indinavir, with HIV protease inhibitors that are currently in clinical use. These included RTV, lopinavir (LPV), and the newer generation protease inhibitors ATV and TPV. Because these protease inhibitors are rapidly metabolized in vivo, they are given in combination with RTV in the clinical setting to prolong their bioavailability. For the purpose of our studies, we examined the effects of exposure to individual protease inhibitors. We have found that in vitro exposure to these protease inhibitors for 48–96 h induces apoptosis of insulinoma and pancreatic islet cells that is reflected by decreases in basal and secretagogue-stimulated insulin secretion. Furthermore, we find a higher incidence of apoptosis in pancreatic islets isolated from rats administered IDV for 3 wk.
An earlier study reported a decrease in glucose-stimulated insulin secretion from mouse islets incubated with several different protease inhibitors, including IDV, for only 10 min, and this was attributed to inhibition of glucose transport function (20). The reversibility of this effect following such acute exposures suggested that the protease inhibitors may contribute to a hyperglycemic state in genetically predisposed individuals. Our findings in the protease inhibitor-administered ZDF rats supports this possibility. In contrast to the findings of Koster et al. (20), a subsequent study (43) reported that glucose plus carbachol-stimulated insulin secretion from isolated rat pancreatic islets exposed to IDV for 48 h was not significantly different from secretion from vehicle-exposed control islets. A potential reason for the discrepancy between the two studies might be related to the differences in insulin secretion profile between mouse and rat islets. In neither of these studies, however, was the viability of islets cells examined.
Similar to the latter study, we also found no difference in insulin-secretory function in human pancreatic islets exposed to protease inhibitors for 72 h, although islet cell apoptosis was evident as early as 24 h. By 96 h, however, glucose-stimulated insulin secretion from the human islets was significantly reduced. These findings raise the possibility that decreases in β-cell secretory capacity following exposure to HIV protease inhibitors may be a result of a combination of β-cell apoptosis and additional independent deleterious effects of the drugs on the secretory pathway. We also found evidence of cell death in pancreatic islets isolated from rats administered a protease inhibitor for 3 wk. Curiously, the islet yield from those animals was minimal, suggesting that either the number of islets was reduced or that the loss of β-cells altered the density of the islets such that they could not be recovered in the gradient fraction expected to contain normal, healthy islets. The recovered islets in fact showed signs of significant damage, as most appeared to have lost their structural integrity. Collectively, the findings under in vitro and in vivo conditions indicate that exposures to protease inhibitors can cause decreases in β-cell viability and function. These effects clearly appear to occur following longer exposures to protease inhibitors than were previously examined (20, 43).
It has been suggested that protease inhibitors possess both antiapoptotic and proapoptotic effects (2), and a recent report suggested that the first-generation protease inhibitor nelfinavir and the newer-generation protease inhibitor ATV induced malignant glioma death by inducing ER stress (34). However, we found no increase in activated PERK in islets from IDV-administered rats or induction of ER apoptotic factor CHOP in INS-1 cells exposed to RTV, LPV, or ATV, although all protease inhibitors induced cleavage of PARP, a caspase-mediated process. These findings suggest that that the HIV protease inhibitors studied induce INS-1 and pancreatic islet cell apoptosis by a mechanism that most likely does not include the ER stress pathway.
Alternatively, apoptosis can also be induced by a pathway involving the mitochondria and several protease inhibitors (nelfinavir, RTV, saquinavir) have been reported to induce mitochondrial damage (3, 38). The likelihood that the intrinsic apoptotic pathway is induced is supported by the finding that exposure to HIV protease inhibitors in the present study led to the activation of mitochondria-associated caspase-9. We further examined this possibility by assessing MitoMP of INS-1 cells following exposure to RTV or ATV. Loss in MitoMP is a hallmark of apoptosis and leads to the release of cytochrome c into the cytosol. We found that RTV and ATV exposures lead to decompensation of INS-1 cell MitoMP and increases in cytochrome c levels in the cytosol. Both of these events are recognized to occur during the induction of mitochondrial apoptotic pathway, suggesting that protease inhibitors activate this pathway.
In summary, our data indicate that several HIV protease inhibitors currently in clinical use induce β-cell apoptosis via the mitochondrial pathway. Although the newer-generation drugs by themselves might appear to have a lower level of β-cell toxicity, the potential advantage of using them is negated by the fact that they are coadministered with RTV, which appears to be very toxic to the β-cells. Our findings therefore suggest that the effects of protease inhibitors on β-cells need to be considered when assessing glucose homeostasis or the development of diabetes in people living with HIV+ and taking HIV protease inhibitors.
GRANTS
This work was supported by grants from NIH (RO1-69455, P01-HL-57278, P60-DK-20579, and P30-DK-56341), T32-DK-007296-27 (to M. J. C), Bristol-Myers Squibb (to K. E. Y), and the Campbell Foundation (S. R).
Acknowledgments
We thank the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, for providing the following protease inhibitors: (ritonavir, reagent 4622); (lopinavir, reagent 9481); (atazanavir, reagent 10003); and (tipranavir, reagent 11285). We also thank the ICR Basic Science Islet Distribution Program and Washington University/Juvenile Diabetes Research Foundation (Award no. 31-2008-382 to Dr. Thalachallour Mohanakumar) for providing (to approved user S.R.) human islets used in these studies. We are also grateful to Dr. Mary Wohltmann for providing expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277: 112–116, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Badley AD In vitro and in vivo effects of HIV protease inhibitors on apoptosis. Cell Death Differ 12: 924–931, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Badley AD, Roumier T, Lum JJ, Kroemer G. Mitochondrion-mediated apoptosis in HIV-1 infection. Trends Pharmacol Sci 24: 298–305, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Korner T, Stoll M, Schmidt RE. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS 13: F63–70, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla AM, Novati R, Calori G, Meneghini E, Vacchini D, Luzi L, Castagna A, Lazzarin A. Stavudine or indinavir-containing regimens are associated with an increased risk of diabetes mellitus in HIV-infected individuals. AIDS 17: 1993–1995, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Carper MJ, Cade WT, Cam M, Zhang S, Shalev A, Yarasheski KE, Ramanadham S. HIV-protease inhibitors induce expression of suppressor of cytokine signaling-1 in insulin-sensitive tissues and promote insulin resistance and type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 294: E558–E567, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12: F51–58, 1998. [DOI] [PubMed] [Google Scholar]
- 8.den Boer MAM, Berbee JFP, Reiss P, van der Valk M, Voshol PJ, Kuipers F, Havekes LM, Rensen PCN, Romijn JA. Ritonavir impairs lipoprotein lipase-mediated lipolysis and decreases uptake of fatty acids in adipose tissue. Arterioscler Thromb Vasc Biol 26: 124–129, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, Meade D, Li XA, Zhu W, Uittenbogaard A, Wilson ME, Smart EJ. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest 111: 389–397, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetzman ES, Tian L, Nagy TR, Gower BA, Schoeb TR, Elgavish A, Acosta EP, Saag MS, Wood PA. HIV protease inhibitor ritonavir induces lipoatrophy in male mice. AIDS Res Human Retroviruses 19: 1141–1150, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 352: 48–62, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Davis B, Sax P, Stanley T, Wilson PW, D'Agostino RB, Grinspoon S. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis 32: 130–139, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr 77: 490–494, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem 279: 55147–55152, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard AA, Floris-Moore M, Arnsten JH, Santoro N, Fleischer N, Lo Y, Schoenbaum EE. Disorders of glucose metabolism among HIV-infected women. Clin Infect Dis 40: 1492–1499, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan VL, Nylen ES. Diabetic ketoacidosis in an HIV patient: A new mechanism of HIV protease inhibitor-induced glucose intolerance. AIDS 13: 1987–1989, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kim RJ, Wilson CG, Wabitsch M, Lazar MA, Steppan CM. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity 14: 994–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes 52: 1695–1700, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee GA, Seneviratne T, Noor MA, Lo JC, Schwarz JM, Aweeka FT, Mulligan K, Schambelan M, Grunfeld C. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS 18: 641–649, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The Group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry 46: 10170–10185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenhard JM, Furfine ES, Jain RG, Ittoop O, Orband-Miller LA, Blanchard SG, Paulik MA, Weiel JE. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. Antiviral Res 47: 121–129, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Max S, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infec Dis 30: S96–S116, 2000. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel ML, Colca JR, Kotagal N, Lacy PE. A subcellular fractionation approach for studying insulin release mechanisms and calcium metabolism in islets of Langerhans. Methods Enzymol 98: 182–200, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform GLUT4 at physiologic concentrations. Aids 16: 859–863, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Mynarcik DC, Combs T, McNurlan MA, Scherer PE, Komaroff E, Gelato MC. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J Acquir Immune Defic Syndr 31: 514–520, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr 25: 312–321, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson DW, Thornberry Caspases: killer proteases NA. Trends Biochem Sci 22: 299–306, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Nolte LA, Yarasheski KE, Kawanaka K, Fisher J, Le N, Holloszy JO. The HIV protease inhibitor indinavir decreases insulin- and contraction-stimulated glucose transport in skeletal muscle. Diabetes 50: 1397–1401, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Noor MA, Parker RA, O'Mara E, Grasela DM, Currie A, Hodder SL, Fiedorek FT, Haas DW. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS 18: 2137–2144, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JMd. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem 273: 33533–33539, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD, The HIVOSI. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 338: 853–860, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Pyrko P, Kardosh A, Wang W, Xiong W, Schonthal AH, Chen TC. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res 67: 10920–10928, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science 308: 1582–1583, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ramanadham S, Song H, Hsu FF, Zhang S, Crankshaw M, Grant GA, Newgard CB, Bao S, Ma Z, Turk J. Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2β that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2β transcript. Biochemistry 42: 13929–13940, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reaven GM Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Roumier T, Szabadkai G, Simoni AM, Perfettini JL, Paulau AL, Castedo M, Metivier D, Badley A, Rizzuto R, Kroemer G. HIV-1 protease inhibitors and cytomegalovirus vMIA induce mitochondrial fragmentation without triggering apoptosis. Cell Death Differ 13: 348–351, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Rudich A, Vanounou S, Riesenberg K, Porat M, Tirosh A, Harman-Boehm I, Greenberg AS, Schlaeffer F, Bashan N. The HIV protease inhibitor nelfinavir induces insulin resistance and increases basal lipolysis in 3T3-L1 adipocytes. Diabetes 50: 1425–1431, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Vigouroux C, Gharakhanian S, Salhi Y, Nguyen TH, Adda N, Rozenbaum W, Capeau J. Adverse metabolic disorders during highly active antiretroviral treatments (HAART) of HIV disease. Diabetes Metab 25: 383–392, 1999. [PubMed] [Google Scholar]
- 41.Woerle HJ, Mariuz PR, Meyer C, Reichman RC, Popa EM, Dostou JM, Welle SL, Gerich JE. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes 52: 918–925, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr 40: 398–403, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarasheski KE, Tebas P, Sigmund C, Dagogo-Jack S, Bohrer A, Turk J, Halban PA, Cryer PE, Powderly WG. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr 21: 209–216, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]