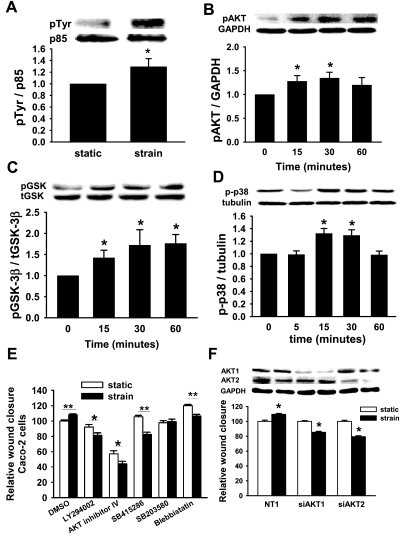
Fig. 1.
Role of PI3K, AKT, glycogen synthase kinase (GSK), and p38 in the motogenic effect of strain. A: strain stimulates phosphorylation of the p85 subunit of PI3K at 30 min in Caco-2 cells (n = 4, *P < 0.03). Phosphorylation of AKT (B), GSK (C), and p38 (D) occurs as early as 15 min of strain (n ≥ 6, *P < 0.02). Inhibiting these signals in Caco-2 cells with the PI3K inhibitor LY294002 (20 μM), AKT inhibitor IV (1 μM), GSK inhibitor SB415286 (20 μM), or p38 inhibitor SB203580 (20 μM) (E) blocked the motogenic effect of strain (solid bars) similarly to nonmuscle myosin II inhibition with blebbistatin (85.5 μM) compared with wound closure in static cells (open bars). Data summarize results of 6–36 holes per experiment from at least 3 independent experiments (*P < 0.05, **P < 0.001). F: isoform-specific siRNA reduced AKT1 or AKT2 78 and 65%, respectively (blots). Reducing either AKT1 or AKT2 reversed the motogenic effect of strain across fibronectin (graph, n ≥ 25, *P < 0.0001). t-, total; p-, phospho; NT1, nontargeting control SiRNA.