Figure 1. Diffusive and directed modes of microtubule based motility.
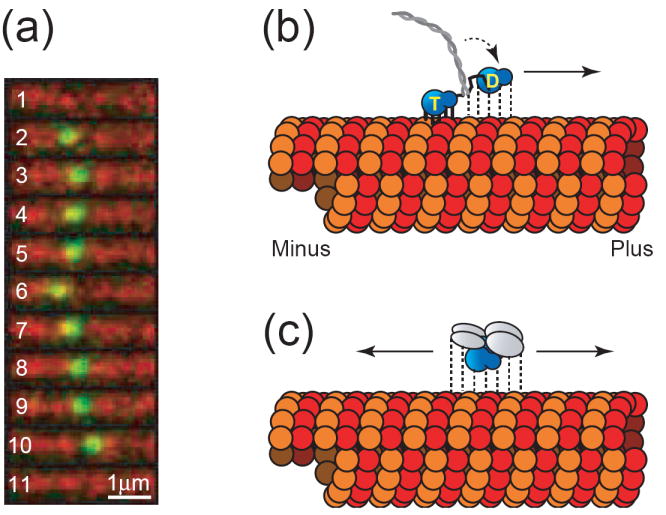
(a) Still frame series exhibiting unbiased diffusional motility of a single MCAK molecule. The molecule binds to the microtubule lattice (frame 2), diffuses 1-dimensionally along the microtubule lattice (frames 2-10), and then detaches (frame 10). Frames 1 and 11 show the microtubule immediately before and immediately after the binding event, respectively. Frame rate is 5 frames per second. GFP-labeled MCAK is shown in green and Cy5-labeled microtubules are shown in red (unpublished data). (b) Model of hand-over-hand directed motility of a kinesin motor. The two motor domain heads alternate between strongly bound (solid lines) and weakly bound states (dashed lines) while hydrolyzing ATP to advance toward the microtubule plus end. The strongly bound head possesses ATP (labeled “T”) in its nucleotide-binding pocket, while the weakly bound head has hydrolyzed its nucleotide to ADP (labeled “D”). During interludes where both motor domain heads possess ADP (not shown), the entire molecule would be weakly bound which can explain the diffusional component sometimes observed superimposed on top of directed motility [9]. (c) Model of unbiased diffusional motility of MCAK. The motor domain is shown in blue while the n-terminal and c-terminal dimerization domains are shown in gray. With either ADP or ATP in its nucleotide-binding pocket, MCAK maintains relatively weak binding to the microtubule lattice [3]. This allows for the molecule to slide randomly in either direction along the microtubule lattice while maintaining its attachment.
