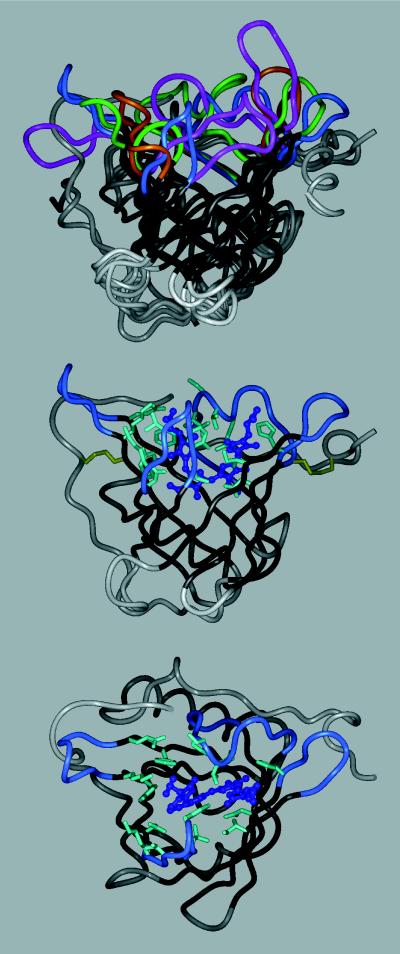
Figure 1.
Tertiary structure of the bilin-binding protein (5), either alone (Middle), with the randomized amino acids depicted, or superimposed (Top) with three other lipocalins: retinol-binding protein, RBP (6); mouse major urinary protein, MUP (7); and epididymal retinoic acid-binding protein, EPA (8). The polypeptide backbone is shown in a ribbon representation (InsightII molecular modeling software), with the β-barrel in black, the four loops surrounding the ligand pocket colored (BBP, blue; RBP, magenta; MUP, green; EPA, amber), and the remainder of the structure in gray. BBP (Middle) is shown with its natural ligand, biliverdin IXγ, in a ball and stick representation (dark blue). The 16 amino acid positions at the center of the binding site that were subjected to random mutagenesis are depicted with their original side chains (light blue). The two disulfide bonds are indicated as well (yellow). (Bottom) The same molecule is shown in an orientation that permits viewing into the binding pocket.