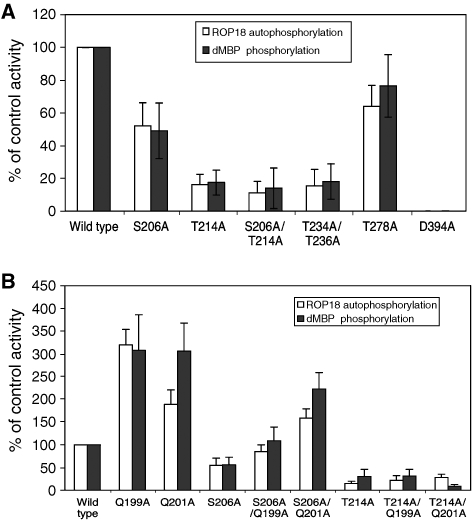
Figure 7.
Mutation analysis of autophosphorylation in the N-terminal extension of ROP18. (A) In vitro kinase reactions of ROP18 autophosphorylation versus transphosphorylation of dMBP by wild-type and mutant ROP18 kinases. Mutation of Ser206A partially blocked activity, whereas mutations of Thr214A or Thr234A/Thr236A lead to greater decreases in activity. Mutation of T278A, which lies in the kinase domain had no effect. For comparison, mutation of D394A, which is in the catalytic triad, abolished all activity. (B) Enhanced activity of ROP18 bearing mutations in Gln199 or Gln201 and influence on restoring activities of Ser206A and Thr214A mutations. Activities were determined by phosphorimager analysis of proteins resolved on SDS–PAGE gels following in vitro kinase reactions. Data are averages±s.d. for three or more similar experiments.