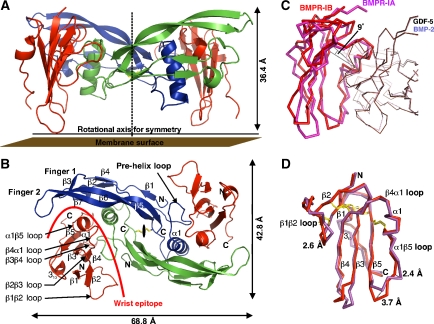
Figure 1.
Architecture of the complex of GDF-5 bound to BMPR-IB. (A) Ribbon representation of the full tetrameric complex of GDF-5 dimer (in blue and green) bound to the extracellular domains of two BMPR-IB molecules (red). A stippled line indicates the crystallographic two-fold axis. (B) As in (A), but viewed from the top. (C) The complex structures of GDF-5:BMPR-IB and BMP-2:BMPR-IA (PDB 1REW) were structurally aligned using the Cα atoms of both ligand dimers and the program Quanta2006. The ligand dimer superposition exhibits an r.m.s.d. of 0.86 Å (Cα of GDF-5: 17–74, 80–120 versus BMP-2: 12–74, 75–114). The ligand superposition clearly reveals that BMPR-IA and BMPR-IB are shifted in both complexes up to 2 Å. Further inspection shows that the BMPR-IB molecule (red) is tilted by 9° (angle between Cys82(BMPR-IB)–Phe66(BMPR-IB)–Cys101(BMPR-IA), see line) towards finger 2 of GDF-5 compared with the orientation of BMPR-IA (magenta) in complex with BMP-2. Residue Phe66 (Phe85 in BMPR-IA) presents the centre of rotation. (D) Despite the reorientation the core structures of both type I receptors are identical (r.m.s.d. 0.7 Å for β-sheet core without β1β2, β3β4 and α1β5 loops), only the β1β2 and α1β5 loops differ significantly.