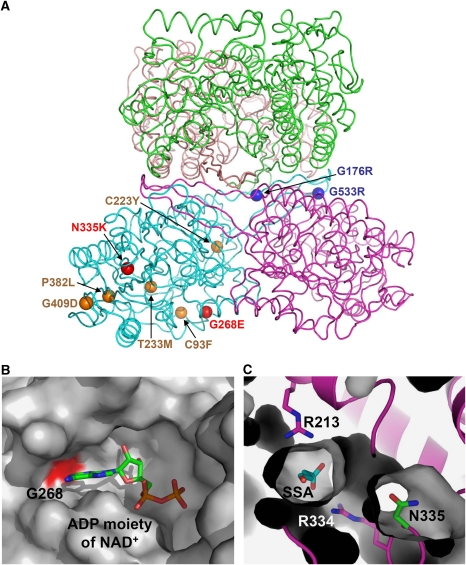
Figure 5.
Impact of patient mutations on the structure and function of human SSADH. (A) The distributions of nine missense point mutations found in SSADH-deficient patients are shown using a ball representation. Mutations that impact on the enzyme catalysis, oligomerization and protein stability are shown and labelled in red, blue and orange colours, respectively. (B) Impact of G268E mutation. The NAD-binding groove is shown as a surface representation in grey colour, and the Gly268 residue, whose mutation to glutamate might interfere the binding of NAD+, is shown in red colour. The ADP moiety of a NAD+ molecule is shown as a stick model and labelled. (C) Impact of N335K mutation. The SSADH–SSA complex is shown as a ribbon diagram in magenta colour, and SSADH excluding the N335 residue is a surface fill model. SSA, N335 and two arginine residues involved in SSA binding are shown as stick models in cyan, green and magenta colours, respectively. The mutation of N335 to lysine might severely distort the active site environment.