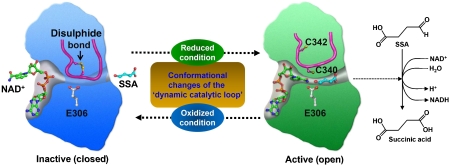
Figure 6.
Redox-switch-mediated enzyme reaction mechanism of human SSADH. SSADH catalyses the conversion of SSA to succinic acid using NAD+ as a cofactor. In oxidized status, the catalytic residue Cys340 is oxidized forming a disulphide bond with an adjacent cysteine residue (Cys342) and the ‘dynamic catalytic loop' is in a ‘closed' conformation, leading to the enzyme inactive. When the environment is switched to reduced status, the disulphide bond is broken, and the ‘dynamic catalytic loop' undergoes structural changes to an ‘open' form in which both SSA and NAD+ are able to access to. Upon breakage of the disulphide bond, the thiol group of Cys340 moves towards a general base Glu306 to form an active site environment.