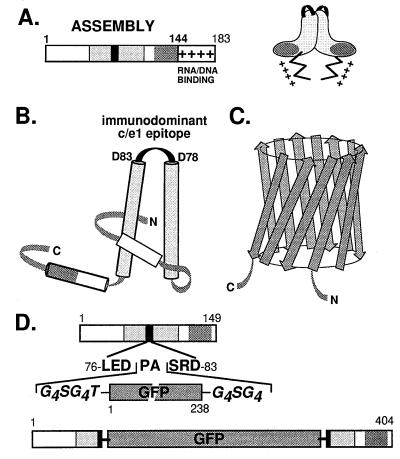
Figure 1.
Structural features of the constituent components of GFPcore1–149 (A) HBV core protein. The bar represents the primary sequence of the full-length core protein c1–183. Within the assembly domain (amino acids 1–144), the c/e1 epitope (black rectangle) and the regions involved in intradimer (light shading) and interdimer (dark shading) contacts are indicated. The Arg-rich nucleic acid binding domain is symbolized by +. A schematic topological view of the dimer is shown on the right. (B) Secondary structure model of the core protein. Cylinders represent α-helices, and the shading corresponds to that in A. The central helices are connected by a short loop overlapping with the c/e1 epitope. In the dimer, these helices form a four-helix bundle (adapted from ref. 22). N and C represent the N and C termini. (C) Schematic representation of GFP. The major secondary structure elements are β-strands forming an 11-stranded β-barrel (35, 36). (D) Chimeric GFPcore1–149 protein. The DNA construct was designed such that the entire GFP sequence, flanked on both sides by Gly-rich linkers, is inserted into the central c/e1 epitope of the truncated, assembly-competent core protein derivative core1–149; the authentic amino acids Pro-79 and Ala-80 are removed. The shading of core protein regions is as in A.