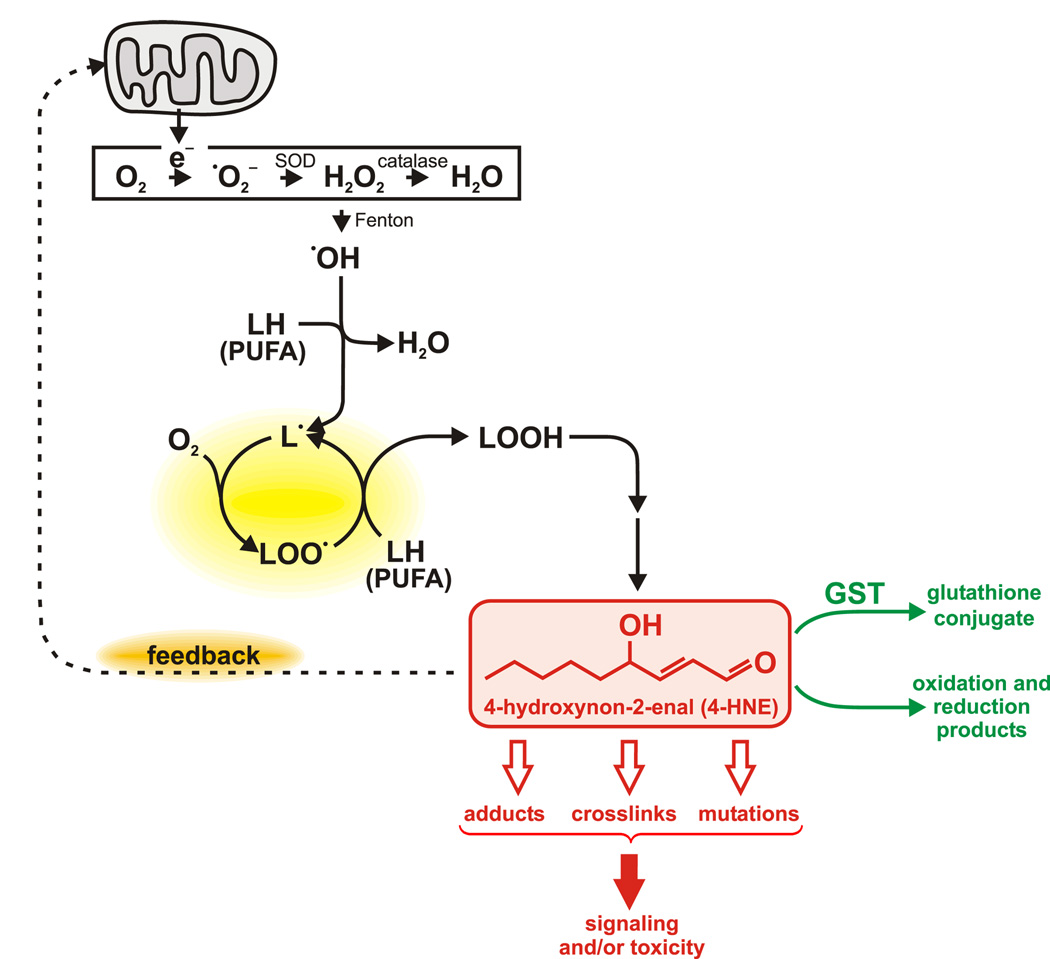
Fig. 1.
Schematic representation of the formation and functions of the lipid peroxidation product 4-HNE. Electrons leaking from the mitochondrial respiratory chain to molecular oxygen lead to the formation of superoxide which can be converted to other ROS. ROS can be generated by other processes (e.g., Krause, 2006) but mitochondria are their major source. Via the Fenton reaction, transition metals catalyze the conversion of H2O2 to the hydroxyl radical which can abstract a hydrogen from a polyunsaturated fatty acid (PUFA) molecule (denoted as LH), generating a carbon-centered radical. The latter initiates a chain reaction provided there is a supply of oxygen and additional PUFAs (yellow highlight). The resulting lipid hydroperoxides (LOOH) can be converted to electrophilic aldehydes such as 4-HNE. These aldehydes are metabolized (green lettering) by glutathione conjugation or redox reactions (reduction of the aldehyde group or of the carbon-carbon double bond, or oxidation of the aldehyde group). Otherwise, 4-HNE can react with cellular macromolecules, chiefly proteins (Fig. 2), changing their activity. This can modulate signaling or cause toxicity. Targets of 4-HNE include mitochondrial proteins, leading to either a negative or positive feedback loop (orange highlight): activation of mitochondrial uncoupling proteins by 4-HNE decreases ROS production (Echtay and Brand, 2007; Echtay et al., 2003; Wolkow and Iser, 2006), but damage to components of the respiratory chain increases generation of ROS (Lee et al., 2006; Uchida, 2003). The latter process could additionally amplify the action of 4-HNE by triggering a higher level of lipid peroxidation. The concentration of 4-HNE may determine whether negative or positive feedback predominates.