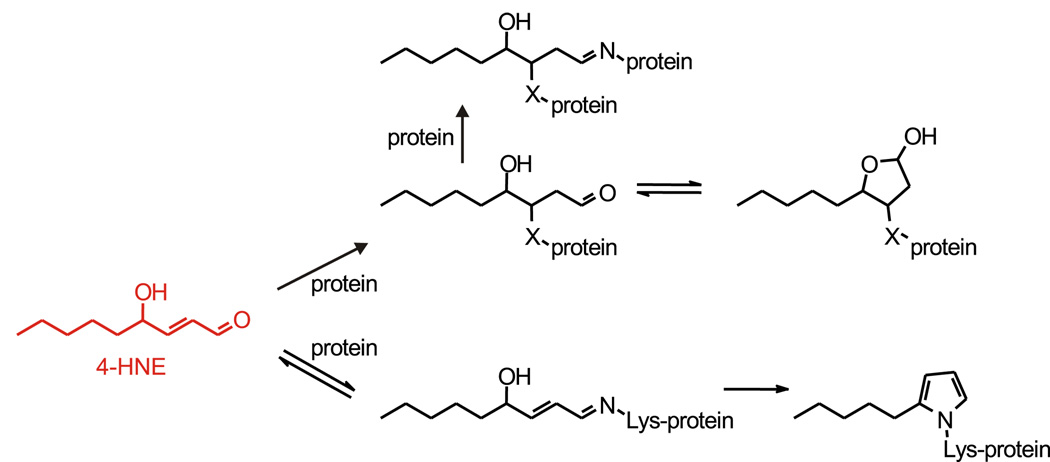
Fig. 2.
Major reactions of 4-HNE with proteins. Upper part of scheme: the predominant reaction is a Michael addition of a nucleophilic center (side chain of cysteine, histidine, or lysine) in a protein to the double bond of 4-HNE. Subsequently, the resulting adduct can cyclize to a hemiacetal, or the aldehyde group of the 4-HNE moiety of the adduct can form a Schiff base with an amino group on the same or another protein, causing protein cross-linking. Lower part of scheme: a less common reaction is the formation of a Schiff base of the aldehyde group of 4-HNE with a lysine side chain in a protein, followed by dehydration and cyclization to a pentylpyrrole adduct. The scheme is based on (Liu et al., 2003).