Abstract
Although it has been well documented that aberrant major histocompatibility complex class II molecules may contribute to the development of autoimmune disorders, the precise mechanisms responsible for their tissue-specific expression remain unknown. Here we show that estrogen deficiency induces aberrant class II major histocompatibility complex expression in exocrine glands via interactions between epithelial cells and plasmacytoid dendritic cells. Relatively modest but functionally significant expression levels of major histocompatibility complex class II and class II transactivator molecules were observed in the exocrine glands of ovariectomized (Ovx) C57BL/6 (B6) mice, but were not seen in the exocrine glands of control B6 mice. We observed that the salivary dendritic cells adjacent to the apoptotic epithelial cells positive for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling, were activated in Ovx mice, but were not activated in control mice. We obtained evidence that the salivary gland cells express both interferon regulatory factor-1 and class II transactivator type IV molecules in Ovx mice. Salivary gland cells from Ovx mice were also capable of inducing the activation of antigen-specific T cells from OT-II transgenic mice. These findings indicate that estrogen deficiency initiates class II transactivator type IV mRNA expression in exocrine glands via interactions between epithelial cells and plasmacytoid dendritic cells, suggesting that plasmacytoid dendritic cells play a pivotal role in gender-based autoimmune disorders in postmenopausal women.
Major histocompatibility complex (MHC) class II molecules are crucial for restricting immune reactivity to self versus foreign antigens during thymic education, and their expression level is essential for determining T cell activation in the periphery.1,2,3 MHC class II molecules are basically expressed on antigen (Ag)-presenting cells of the hematopoietic lineage, but can be induced by unknown stimuli on many other cell types (such as endothelial cells, hepatocytes, pancreatic β-cells, thyrocytes, and salivary gland cells) in association with autoimmunity.4,5,6,7,8,9,10 MHC class II proteins play a central role in the control of normal immune homeostasis, while aberrant expression of MHC class II is frequently associated with abnormalities in immune responses.11,12,13 MHC class II expression is regulated by multiple cytokines, including interferon (IFN)-γ, which can enhance the immune response by upregulating MHC class II expression in immune cells, in addition to inducing Ag-presentation capacity.14,15 It is well known that the induction of MHC class II gene transcription by IFN-γ is mediated by the MHC class II transactivator (CIITA), and depends on the presence of two transcription factors; signal transducer and activator of transcription 1 and interferon regulatory factor (IRF-1).16 CIITA is a true co-activator because it does not bind directly to DNA but mediates its function on the class II MHC promoter through interaction with other proteins.17,18 It is considered a master regulator because it is necessary and sufficient for the expression of all of the genes in the MHC class II pathway. Although recent studies have begun to reveal that epigenetic mechanisms have a key role in activating CIITA expression in normal cells,19 the factors regulating this promoter have yet to be investigated.
Physiological gender differences in the immune system are well recognized and suggest that sex steroid hormones such as estrogens may be involved in the regulation of the immunocompetence.20,21,22,23,24 Estrogenic action has been suggested to be responsible for the strong female preponderance of many autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome (SS).25,26 Previously, we demonstrated that estrogenic action influences target epithelial cells through Fas-mediated apoptosis in a murine model of SS.27 Moreover, we found that the tissue-specific apoptosis in the exocrine glands spontaneously occurring in estrogen-deficient healthy C56BL/6 (B6) mice may contribute to the development of autoimmune exocrinopathy resembling SS.28 Recently in minor salivary glands from patients with SS, significantly increased development of IFN pathways and increased plasmacytoid dendritic cells (pDCs) as a possible source of IFN were shown.29 The persistence of the IFN signature might be related to a vicious circle in the pathogenesis of autoimmune diseases. Despite extensive characterization of pDCs, there are still unsolved mysteries regarding their origin and function.
In this study, we have focused on the molecular mechanisms responsible for tissue-specific CIITA expression caused by estrogen deficiency, resulting in aberrant expression of MHC class II molecules in the exocrine glands.
Materials and Methods
Mice and Treatments
Female B6 mice (H-2b) were purchased from Japan SLC (Shizuoka, Japan), and maintained in a specific pathogen-free mouse colony and given food and water ad libitum. Mice were ovariectomized (Ovx group) at 4 weeks of age and compared with sham-operated (Sham) mice. At 1 to 5 weeks after ovariectomy, all organs were removed from the mice and analyzed. To examine the antigen-presenting activity, ovalbumin (OVA)-specific T cell receptor-transgenic OT-II mice30 (C57BL/6-Tg [TcraTcrb]425Cbn/J) obtained from S. Webb (The Scripps Research Institute, La Jolla, CA), were used. All of the experiments were approved by the animal ethics board of Tokushima University.
Preparation and Primary Culture of Mouse Salivary Glands Suspension Cells
Mouse salivary gland suspension (SGS) cells were prepared as previously described.27,28 Briefly, mouse submandibular glands were minced into 1-mm2 pieces, digested with collagenase (750 U/ml of type I) and hyarulonidase (500 U/ml of type IV) in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. The digested suspension was passed through a 75-μm nylon mesh filter. SGS number were counted and seeded at 2 × 105/ml with Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum in a collagen-coated dish. After 4 hours, culture medium was changed to HuMedia-KB2 (Kurabo, Osaka Japan) and was incubated in 5% CO2 atmosphere at 37°C. Over the next 4 to 7 days the original cultures using the KB2 medium were cultured with phenol red-free Dulbecco’s modified Eagle’s medium containing 2% charcoal-stripped fetal calf serum for 24 hours. The primary cultured SGS cells were treated with (E2) (10 pM) (Wako Pure Chemical Ltd., Osaka), tamoxifen (10 μM) (MP Biomedicals, Burlingame, CA) with or without IFN-γ (4 ng/ml) (eBioscience, San Diego, CA) for 4 hours. Finally, the total RNA was prepared from these treated cells for quantitative RT-PCR.
Flow Cytometric Analysis
Surface markers of spleen cells and SGS cells were identified with monoclonal antibodies (mAbs) using FACSCanto flow cytometer (Beckman Coulter, Inc., Miami, FL). Fluorescein isothiocyanate (FITC)-conjugated rat mAb to MHC Class II and phycoerythrin (PE)-conjugated rat mAbs to CD86 and B220, and allophycocyanin (APC)-conjugated hamster mAb to CD11c (eBioscience, San Diego, CA) were used. Epithelial cells were stained with rabbit anti-mouse keratin Abs (Cosmo Bio CO. LTD, Tokyo) as primary Ab and PE-conjugated anti-rabbit IgG as secondary Ab. Appropriate isotype-matched controls were used. Data were analyzed with FlowJo FACS analysis software (Tree Star, Ashland, OR).
Confocal Microscopic Analysis
The frozen sections of salivary and lacrimal glands from Ovx- and Sham B6 mice were fixed with cold acetone, then blocked with M.O.M.™ blocking reagent (Vector Laboratories, Inc. Burlingame, CA), and stained with following antibodies: PE-conjugated antibodies to EpCAM, PDCA1, CD86, and CD11c; biotinylated antibodies to CD11c. The secondary detection reagents included FITC-conjugated antibodies to MHC class and CD11c; Alexa Fluor 488-conjugated antibody to rat IgG (H+L), Alexa Fluor 568-conjugated streptavidin, and Alexa Fluor 488-conjugated anti-FITC IgG (Molecular Probes Inc., Eugene, Oregon).
The nuclear DNA was stained with 4′,6-diamidino-2-phenylindole dihydrochloride (Molecular Probe Inc.). The images were acquired with LSM 5 PASCAL Confocal Laser-scanning microscope (Carl Zeiss, Germany). For quantitative analysis of sections, positive cells and 4′,6-diamidino-2-phenylindole-stained nuclei were counted in 10 areas of the microscopic slides at a magnification of ×630. The value was presented as the percentage of the number of positive cells to the total number of nuclei.
Quantitative Reverse Transcription PCR Analysis
Total RNA was extracted from primary cultured SGS and tissue using ISOGEN (Wako Pure Chemical, Osaka, Japan), and reverse transcribed. Transcript levels of MHC class II, IRF-1, CIITA, and β-actin were observed using PTC-200 DNA Engine Cycler (BioRad) with SYBR Premix Ex Taq (Takara, Kyoto, Japan). The following primer sequences were used: for MHC class II, 5′-GCCTCTGCGGAGGTGAAGA-3′ (forward) and 5′-CAAGTCCACATAGAACAACTCATCACC-3′ (reverse); for IRF-1, 5′- AACTCCAGCACTGTCACCGTG-3′ (forward) and 5′- GAGTTGCCCAGCAGGCTGTCC-3′ (reverse): for total CIITA, 5′- TGCAGGCGACCAGGAGAGACA-3′ (forward) and 5′- GAAGCTGGGCACCTCAAAGAT-3′ (reverse); for type I CIITA, 5′-AAGAGCTGCTCTCACGGGAAT-3′ (forward); for type III CIITA, 5′-TCTTACCTGCCGGAGTT-3′ (forward); for type IV CIITA, 5′-GAGACTGCATGCAGGCAGCA-3′ (forward); for type1, type III, and type IV CIITA reverse, GGTCGGCATCACTGTTAAGGA; and for β-actin, 5′- GTGGGCCGCTCTAGGCACCA-3′ (forward) and 5′-CGGTTGGCCTTAGGGTTCAGGGGGG-3′ (reverse). Relative mRNA abundance of each transcript was normalized against β-actin.
Enzyme-Linked Immunosorbent Assay
The amount of mouse interleukin (IL)-2 and IL-10 in culture supernatants from CD4+ T cells and SGS cells were analyzed by enzyme-linked immunosorbent assay (ELISA). Briefly, immunoplates were coated with anti-cytokine capture antibody, and washed with PBS/0.1% Tween 20. After blocking with 2% bovine serum albumin in PBS, the plates were incubated with diluted culture supernatants or standards. The plates were washed and incubated with biotin-conjugated antibodies for cytokine detection and a horseradish peroxidase-conjugated streptavidin was added. Color reaction was developed with o-phenylendiamine (Sigma Chemical Co., St. Louis, MO) in 0.1 M citrate buffer (pH5.0) containing 0.01% H2O2. Rat anti-mouse IL-2 mAb (JES6-1A12, eBioscience), biotinylated rat anti-mouse IL-2 mAb (JES6-5H4, eBioscience), and recombinant mouse IL-2 standard (eBioscience) were used for IL-2 ELISA. Rat anti-mouse IL-10 (JES5-16E3, eBioscience), biotinylated rat anti-mouse IL-10 mAb (LES5-2A5, eBioscience), and recombinant mouse IL-10 standard (eBioscience) were used for IL-10 ELISA. The detection limits of cytokine for each were; 5 pg/ml for IL-2, 40 pg/ml for IL-10.
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
Apoptotic cells were detected in sections using the in situ apoptotic detection kit (Wako Pure Chemical Industries) according to the manufacture’s instructions. Frozen sections were fixed with 3% paraformaldehyde and incubated in labeling reaction mixture (consisting of TdT Enzyme and Labeling Safe Buffer containing fluorescein-labeled nucleotides) for 90 minutes at 37°C. For the double staining of CD11, the sections were incubated with biotin blocking system (Dako North America, Inc, Carpinteria, CA) to block endogenous biotin, and blocked with Vector M.O.M. blocking reagents (Vector, Burlingame, CA), stained with the biotinylated hamster anti-CD11c mAb, and finally, visualized with Alexa Fluor 568-conjugated streptavidin.
Proliferation Assay of OT-II CD4+ T Cells to the SGS Cells from Ovx-B6 Mice
OT-II CD4+ T cells from OT–II transgenic mice for OVA-specific T cell receptor were isolated from spleen cells by negative depletion using a mixture of anti-CD8, anti-B220, and anti-CD25 mAbs, followed by anti-rat IgG conjugated magnetic beads (Dynal, Olso, Morway). Enriched CD4+ T cells (105) were cultured with irradiated (7 Gy) SGS cells or CD11c-depleted SGS cells at the appropriated ratio in round-bottom 96-well plates. Cells were treated with 10 μg/ml OVA peptide, amino acids 323 to 339 (Abgent, San Diego CA), for 72 hours, and were pulsed with 3H-thymidine during the final 8 hours of culture, and 3H-thymidine incorporation was assayed with an automated liquid scintillation counter.
Isolation of DCs and Stimulation
For flow cytometric analysis, SGS derived dendritic cells were prepared from SGS cells in Sham and Ovx mice by negative selection with anti-EpCAM mAb and anti-rat IgG conjugated magnetic beads. For stimulation with UV-induced apoptotic epithelial cells, salivary gland DCs were purified by positive selection using CD11c microbeads (Miltenyi Biotec). Freshly isolated CD11c+ cells were stimulated with primary cultured SGS cells with or without the UV irradiation (5 minutes.) for 48 hours. Cytokine concentration of the culture medium was assayed by ELISA for IL10.
Effects of in Vivo Administration of IFN-γ
To examine the in vivo effect of IFN-γ administration, 0.5 μg recombinant mouse IFN-γ (eBioscience) in 100 μl PBS was injected intravenously into Sham or Ovx B6 mice. At 3 hours after the injection all of the organs were removed, and then the total RNA was extracted for analyzing the expression of IRF-1 and CIITA by quantitative reverse transcription (RT)-PCR.
Statistical Analysis
The results were expressed as means ± SD with at least in triplicates per group. Values of p were determined using Student’s t-test.
Results
Tissue-Specific MHC Class II Expressions in the Exocrine Glands of Estrogen-Deficient Mice
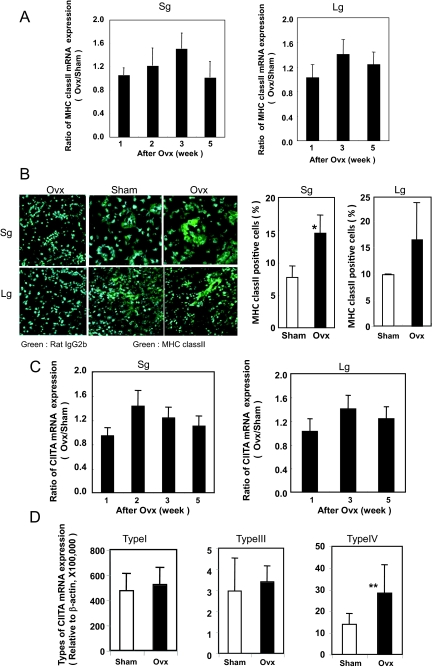
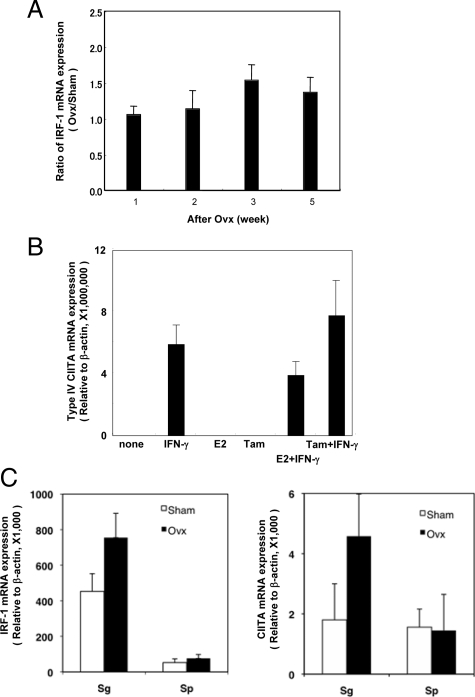
To examine the in vivo expression of MHC class II in estrogen deficient B6 mice, ovariectomy was performed at the age of 4 weeks. Increased MHC class II mRNA expression in the salivary and lacrimal glands at 3 weeks after ovariectomy (7 weeks old) was detected, but declined with age by 8 weeks (12 weeks old) as determined by quantitative RT-PCR analysis (Figure 1A). No change in MHC class II expression was observed in other organs of Ovx-B6 and Sham-B6 mice (data not shown). We detected a distinct MHC class II expression in salivary and lacrimal gland duct cells at 3 weeks after ovariectomy (7 weeks old) by confocal analysis, as compared with negligible expression in Sham-B6 mice (*P < 0.05) (Figure 1B). The expression of MHC class II molecules is regulated generally at the transcriptional level, including the transcription factor CIITA, the master regulator for MHC class II gene expression.17,18,31,32 In Ovx-B6 mice, significant CIITA mRNA expression in the salivary and lacrimal gland tissues was observed respectively at 2 weeks and 3 weeks after ovariectomy, and later declined with age by quantitative RT-PCR analysis (Figure 1C). CIITA has three different isoforms synthesized from different, cell-specific promoters33; promoter I is mainly used in dendritic cells, promoter III in B lymphocytes, and promoter IV in nonhematopoietic cells. The CIITA expression was controlled by each promoter referred to as type I, III, and IV. Using the type specific CIITA primers, a significantly increased expression of type IV mRNA, not type I or type III mRNA, was observed in the salivary glands from Ovx-B6 mice, compared with those from Sham-B6 mice (*P < 0.002)(Figure 1D).
Figure 1.
Tissue-specific MHC class II and CIITA expressions in the exocrine glands of estrogen-deficient mice. A: An increased MHC class II mRNA expression in the salivary and lacrimal glands was detected at 3 weeks after Ovx (7 weeks old), but later declined with age on quantitative RT-PCR analysis. Data represents the ratio of the relative MHC class II mRNA expression value, normalized with β-actin, from OVX-B6 mice to the average of those from Sham-B6 mice at the each age (n = 5 to 8). Sg: salivary gland; Lg: lacrimal gland. B: MHC class II expression was observed in the salivary and lacrimal epithelial duct cells at 3 weeks after Ovx (7 weeks old) on confocal analysis, while negligible expressions were observed in Sham-B6 mice. Salivary and lacrimal gland sections form sham- and Ovx-B6 mice were stained with FITC-conjugated anti-MHC class II and then stained with Alexa Fluor 488-conjugated anti-FITC IgG as second Ab. As negative control, FITC-conjugated rat IgG2b were used. The nuclei were stained with 4′,6-diamidino-2-phenylindole. Original magnification ×630. Calculated data has a mean of ± SD of MHC class II-positive cells (percent) of four to six mice. *P < 0.01. C: A significant expression of CIITA mRNA in the salivary and lacrimal gland tissues from Ovx-B6 mice were observed on quantitative RT-PCR analysis in 2 to 3 weeks after Ovx, and later declined with age, but not in Sham-B6 mice. D: A significantly increased expression of CIITA type IV mRNA, not CIITA type I or CIITA type III mRNA, was observed in the salivary glands at 3 week after Ovx, compared with those from Sham-B6 mice (n = 8). **P < 0.002.
Increased pDCs in the Exocrine Glands of Estrogen-Deficient Mice
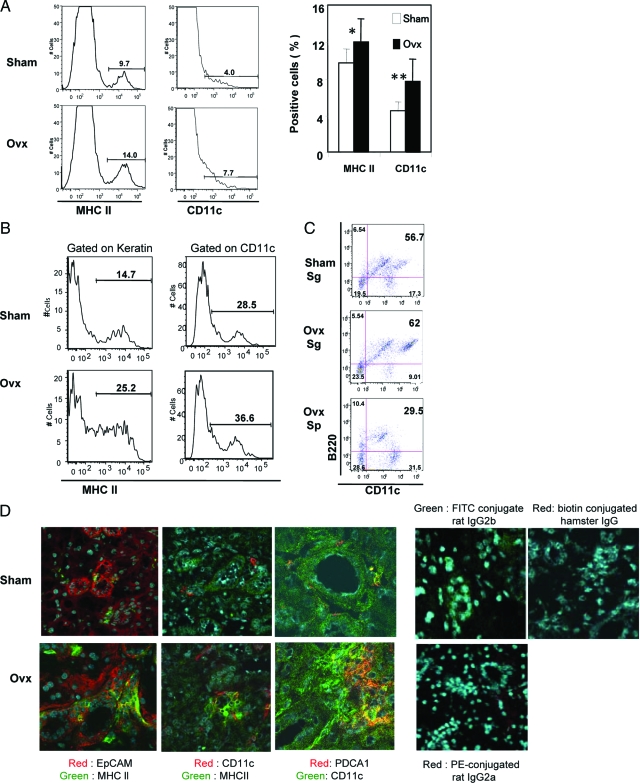
We next examined by flow cytometry whether SGS cells from Ovx-B6 mice could express MHC class II and CD11c molecules. An increased proportion of MHC class II+ and CD11c+ cells was observed in SGS cells from Ovx-B6 mice, compared with those from Sham-B6 mice (*P < 0.05 and **P < 0.002) (Figure 2A). Keratin+ and CD11c+ SGS cells from Ovx-B6 mice expressed significantly greater levels of MHC class II molecule compared with those from Sham-B6 mice (Figure 2B). It was confirmed after depletion of epithelial cells with anti-EpCAM antibody that these CD11c+ DC enriched from SGS cells, were positive for B220 by flow cytometry (Figure 2C), implying that the salivary gland DCs were pDCs. The population of salivary gland B220+CD11c+ cells from Ovx-B6 mice was significantly larger than that in the spleen, and expressed the higher intensity of CD11c. In addition to the epithelial duct cells positively stained with MHC class II, a lower number of CD11c+ dendritic cells adjacent to the ducts was also positive for class II within the salivary glands from Ovx-B6 mice (Figure 2D). Confocal analysis also demonstrated that the salivary CD11c+ cells expressed PDCA1, a specific pDCs marker, from Ovx-B6 mice (Figure 2D). When the existence of pDCs in the salivary glands were searched for in normal conditions, a smaller number of pDCs in the normal salivary glands from Sham-B6 mice was found than from Ovx-B6 mice, indicating that estrogen deficiency led to increase in the number of pDCs in the salivary glands. These data strongly suggest that estrogen deficiency plays an important role of aberrant MHC class II expression on the exocrine gland through pDCs in vivo.
Figure 2.
Increased plasmacytoid DCs (pDCs) in the exocrine glands of estrogen- deficient mice. A: An increased proportion of MHC class II+ and CD11c+ cells was observed in salivary gland suspension (SGS) from Ovx-B6 mice compared with those from Sham-B6 mice on flow cytometry. Data are representative of five independent experiments. Calculated data have a mean of ± SD of MHC class II-, and CD11c-positive cells (percent) of four to six mice. *P < 0.05, and **P < 0.002. B: SGS cells from Ovx-B6 mice could express significantly increased levels of MHC class II gated on keratin+, and on CD11c+ compared with those from Sham-B6 mice. C: The CD11c+ DC in the salivary glands, enriched by the depletion of epithelial cells with anti-EpCAM Abs, were positive for B220 on flow cytometry, implying that the salivary gland DCs were plasmacytoid (pDCs). Sp; spleen. The population of salivary B220+CD11c+ cells from Ovx-B6 mice was significantly larger than that in the spleen, and expressed the higher intensity of CD11c. Figures are representative of five to eight mice. D: Confocal analysis demonstrated, in addition to the epithelial duct cells positively stained with MHC class II, a lesser number of CD11c+ dendritic cells adjacent to the ducts were also positive for MHC class II within the salivary glands from Ovx-B6 mice. The presence of PDCA1+ pDCs in the salivary glands from Ovx-B6 mice was frequently observed, but not from Sham-B6 mice. FITC conjugated rat IgG2b, biotin conjugated hamster IgG, and PE-conjugated rat IgG2a were used as negative control. Representative photos of five samples are shown.
Activation of DCs in the Salivary Glands through Epithelial Cell Apoptosis
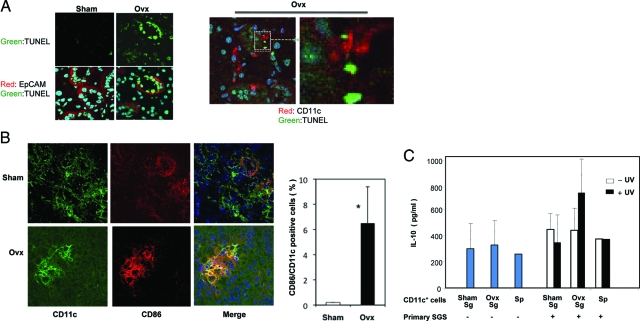
A previous paper stated that significant apoptosis in the salivary gland cells was induced in estrogen-deficient B6 mice,28 indicating that anti-estrogenic action seems to play an important role for epithelial cell apoptosis in vivo. Apoptosis was clearly detected in a significant number of DCs adjacent to the apoptotic epithelial duct cells positive for terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL+) in the salivary glands from Ovx-B6 mice, and not from Sham-B6 mice (Figure 3A). We found frequently that these apoptotic epithelial cells existed closely to CD11c+ DCs in the salivary glands from Ovx-B6 mice (Figure 3A). Moreover, it confirmed the salivary gland DCs from Ovx-B6 mice were activated and expressed CD86, but not DCs from Sham-B6 mice (*P < 0.01) (Figure 3B). Next we examined the effect of UV-induced apoptotic stimuli of primary cultured salivary epithelial cells on the production of IL-12p40, and IL-10 by salivary gland DCs. The increased production of IL-10, not IL-12p40, induced by the co-culture of apoptotic epithelial cells and DCs in the salivary glands from Ovx-B6 mice, was detected (Figure 3C). These results indicate that estrogen deficiency induces the apoptosis of epithelial duct cells, and there is a possible connection between the epithelial cells and DCs in the salivary glands.
Figure 3.
Activation of DCs in the salivary glands via epithelial cell apoptosis. A: The TUNEL+-apoptotic epithelial duct cells (EpCAM+) were frequently detected in the salivary glands from 3 weeks after Ovx, but not from Sham-B6 mice. A large number of CD11c+ DCs adjacent to the TUNEL+-apoptotic epithelial duct cells were detected in the salivary glands from Ovx-B6 mice. The cell-to-cell interaction between these apoptotic epithelial cells and CD11c+ DCs was observed in the salivary glands from Ovx-B6 mice. Representative photos of five samples from each group are shown. B: The salivary DCs from Ovx-B6 mice were activated, expressing CD86, but not in Sham-B6 mice. Calculated data are the mean of ± SD of CD11c-positive cells (percent) of five mice. *P < 0.01. C: IL-10 production was detected in the co-culture medium of UV-induced apoptotic primary cultured epithelial cells and CD11c-positive cells in the salivary glands with ELISA. Data (pg/ml) have means of ± SD of triplicate samples and are representative of four independent experiments. Sp; spleen.
Antigen Presenting Function of the SGS Cells from Ovx-B6 Mice to OT-II Transgenic Mice-Derived T Cells
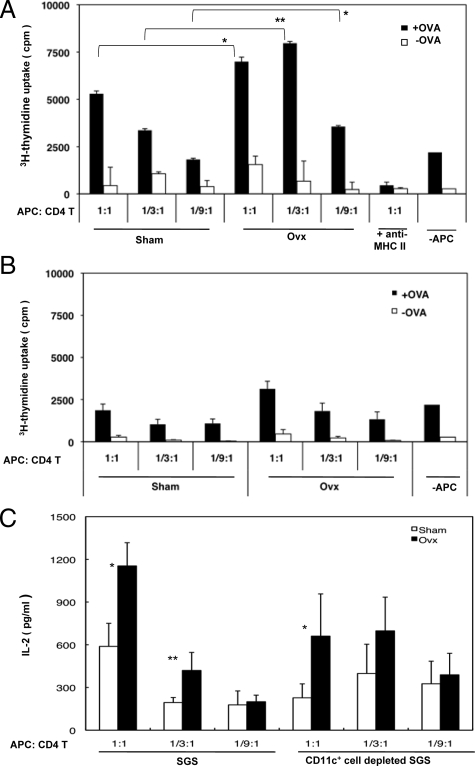
To determine whether MHC class II-expressing salivary gland cells induced by estrogen deficiency may function as antigen-presenting cells, we performed an in vitro proliferation assay using CD4+ T cells from OT-II Tg mice. The enriched CD4+ (1 × 105) T cells from OT-II Tg mice were capable of proliferating to irradiated SGS cells pulsed with OVA peptide from Ovx-B6 mice, compared with those from Sham-mice (Figure 4A). As CD11c-depletion of SGS cells with anti-CD11c mAbs inhibited these responses, most of the activity of antigen-specific T cell proliferation in estrogen-deficient salivary gland cells was attributed to CD11c+ DCs (Figure 4B). Moreover, significant IL-2 production was detected in the culture medium of this condition (Figure 4C). These data suggest that MHC class II-expressing salivary gland cells induced by estrogen deficiency can function as antigen-presenting cells to stimulate antigen-specific T cell response.
Figure 4.
Antigen-presenting function of the salivary gland suspension (SGS) cells from Ovx-B6 mice to OT-II transgenic mice-derived T cells. A: In vitro proliferation assay using CD4+ T cells from OT-II Tg mice with SGS cells. The purified CD4+ (1 × 105) T cells from OT-II Tg mice were capable of proliferating to SGS cells pulsed with OVA peptide from Ovx-B6 mice, compared with those from Sham-mice, while anti-MHC class II antibody (10 μg/ml) inhibits these responses. *P < 0.01 and **P < 0.001. B: The purified CD4+ (1 × 105) T cells from OT-II Tg mice could not proliferate to SGS cells pulsed with OVA peptide from Ovx-B6 mice when the CD11c-positive cells were depleted with anti-CD11c antibody. Data are means ± SD of triplicates. C: A significantly increased IL-2 production was detected by ELISA in culture medium of this condition. IL-2 production was not detected without OVA. Data (pg/ml) are means ± SD of triplicate samples and are representative of four independent experiments. *P < 0.05 and **P < 0.001.
Up-Regulation of IRF-1, and Type IV CIITA mRNA in Estrogen-Deficient Salivary Glands
The expression of MHC class II molecules is also regulated generally at the transcriptional level, including by the transcription factor IRF-1.34 Transcription of CIITA induces transcription of IRF-1. Significantly increased expression of IRF-1 mRNA in the salivary gland tissues from Ovx-B6 mice was observed, but not in Sham-B6 mice (Figure 5A). It has been recently reported that the transcription factor IRF-1 mRNA expression is induced by ICI 182,780 and repressed by estrogens in anti-estrogen–sensitive cells.35 In in vitro studies using primary cultured SGS cells, we detected an increased type IV CIITA mRNA expression when treated with anti-estrogenic tamoxifen plus IFN-γ, as compared with IFN-γ alone. Estrogen (E2) decreased the type IV CIITA mRNA expression amplified with IFN-γ (Figure 5B). Recent studies have begun to reveal that epigenetic mechanisms have a key role in activating CIITA expression in normal cells,19,36 but the regulating factors of this promoter are still unclear. These results suggest that tissue-specific CIITA expression caused by estrogen deficiency led to aberrant expression of MHC class II molecules in the salivary glands through the IRF-1 pathway.
Figure 5.
Upregulation of IRF-1 and CIITA expression in the salivary glands of estrogen-deficient mice, IFN-γ administrated mice and primary cultured SGS cells. A: Significantly increased mRNA expression of IRF-1 in the salivary gland tissues from Ovx-B6 mice was observed, compared with those from Sham-B6 mice. Data (Ovx/Sham ratio) are representative of five independent experiments. B: In in vitro studies using primary cultured SGS cells, an increased expression in typeIV CIITA mRNA was detected when treated with tamoxifen (Tam) plus IFN-γ compared with treatment with IFN-γ alone. Data (relative to β-actin) has a mean of ± SD of triplicate samples and are representative of four independent experiments. C: An increased responsiveness of IRF-1 and CIITA mRNA was observed in the salivary glands by recombinant IFN-γ administration to Ovx-B6 mice than those in Sham-B6 mice, which is likely to be related to a priming effect of IFN-γ characteristic of Ovx-B6 mice. Data (relative to β-actin) are means ± SD of triplicate samples.
In Vivo Effects of IFN-γ Administration for IRF-1and CIITA mRNA Expression in Target Organs by Estrogen Deficiency
Next, the in vivo responsiveness of IRF-1 and CIITA mRNA to recombinant IFN-γ (0.5 μg) administration in the salivary glands due to estrogen deficiency was examined. This confirmed an increased responsiveness of IRF-1 and CIITA mRNA in the salivary glands to IFN-γ administration in Ovx-B6 mice compared with those in Sham-B6 mice (Figure 5C), which is likely related to a priming effect of IFN-γ characteristic of Ovx-B6 mice. Indeed, such phenomenon can be enhanced by the dose-dependent stimulation with IFN-γ in the exocrine glands of Ovx-B6 mice rather than those in the other organs in response to IFN-γ (data not shown), which may contribute to the increased IRF-1 and CIITA mRNA levels in an estrogen-deficient condition.
Discussion
MHC class II molecules are central to the maintenance of self-tolerance and to the breaking of this tolerance during the initiation and development of autoimmune diseases. Autoimmune diseases are more common in females25,26 and the sex hormones, including estrogen, which modulates the immune response,20,21,22,23,24 have an important role in this gender bias. Other studies have proposed hyperprolactinemia as being involved in autoimmunity,37 as well as low dehydroepiandrosterone sulfate levels observed in patients with SS.38 This study demonstrates the first evidence that gender-based, tissue-specific CIITA and MHC class II expression could be induced in the exocrine gland cells via epithelial cells and pDC interaction. MHC class II molecules are primarily expressed on Ag-presenting cells of the hematopoietic lineage such as B cells, dendritic cells, and macrophages besides thymic epithelial cells.1,2,3 Once immune recognition has begun, the aberrant expression of MHC class II on nonprofessional Ag-presenting cells can facilitate disease progression and yield a more severe disease.39,40 Aberrant expression of MHC class II molecules has been revealed in response to IFN-γ, IL-4, and IL-10.18,41 In the present study using normal B6 mice, a relatively modest but functionally significant increase in type IV CIITA, and MHC class II expression in the salivary and lacrimal gland epithelial cells at 3 weeks after ovariectomy was detected, and thereafter declined with age, indicating that the steroid-related hormonal compensation occurred in vivo.
The expression of MHC class II molecules on the nonprofessional APC has been implicated in immune-mediated inflammation and progression, or resistance to autoimmunity.42 However, little is known about the pathways and molecules involved in the regulation of MHC class II Ag presentation and their potential roles in Ag presentation to CD4+ T cells in vivo. These observations raised the question of whether nonhematopoietic cells can function as Ag-presenting cells and whether they are required for damage to the target organ. In this study, it was observed that the purified CD4+ T cells from OT-II Tg mice were capable of proliferating to SGS cells from Ovx-B6 mice pulsed with autopeptide. Thus, it is possible that MHC class II-expressing exocrine gland cells caused by estrogen deficiency can function as Ag-presenting cells toward Ag-specific T cells in vivo. DCs are crucial for the pathogenesis of autoimmune disease because of their potent antigen-presenting activity and unique ability to activate naïve T cells.43 DCs have also been described to prime autoreactive T cells and induce the local inflammation of the synovial membrane in arthritis.44 In addition, antigen-pulsed DCs have been shown to induce disease in experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis.45 In this study, surprisingly, an increased B220+CD11c+ pDCs in the salivary glands in Ovx-B6 mice was found. The percentage of MHC class II and CD86 expression in DCs was significantly increased in Ovx-B6 mice compared with that in Sham-B6 mice. These data suggests that estrogen deficiency increases the number and activity of the DCs in the salivary glands, and that the expansion of pDCs may play a possible role for development of gender-based autoimmune exocrinopathy in postmenopausal women. Indeed, it has been recently reported that pDCs are present in the salivary glands of patients with SS but absent in controls.29 Activation of pDCs links innate to adaptive immunity, leading to increased secretion of type I IFN (IFN-α/β) and IL-12, which promotes type II IFN (IFN-γ) secretion by T cells, natural killer cells, and DCs.45 Thus, the presence of pDCs in the salivary glands of patients with SS sheds light on the pathogenesis of the disease.29 Since the phenomenon in the present study is limited to rodents, further investigation is required to elucidate the physiological significance of salivary pDCs in normal condition.
Our previous reports show tissue-specific apoptosis associated with an elevated caspase-1 and caspase-3 activity in the salivary glands from Ovx-B6 mice.28 In this study, a significant number of pDCs adjacent to the TUNEL+-apoptotic epithelial duct cells in the salivary glands from Ovx-B6 mice were clearly detected. Since relatively modest but functionally significant MHC class II expression in the salivary epithelial cells was observed, it has been suggested that estrogen deficiency plays an important role of aberrant MHC class II expression on the exocrine epithelial cells through activated pDCs in vivo. The present study provides evidence a novel regulatory link between the immune system and sex steroid homeostasis, and may help to explain the tissue-specific autoimmunity in postmenopausal women.
Taken together, although the role of aberrant MHC class II in autoimmune disease development is controversial, estrogen deficiency may play a pivotal role for the induction of aberrant class II molecule in the exocrine glands. Thus, recognition of the immune targets of estrogen in vivo may lead to identification of new therapeutic interventions for autoimmune conditions in the exocrine glands.
Footnotes
Address reprint requests to Yoshio Hayashi, Department of Oral Molecular Pathology, Institute of Health Biosciences, The University of Tokushima Graduate School, 3 Kuramotocho, Tokushima 770-8504, Japan. E-mail: hayashi@dent.tokushima-u.ac.jp.
Supported in part by a Grant-in-Aid for Scientific Research (Nos. 17109016, 18390498, and 20390479) from the Ministry of Education, Science, Sports, Technology, and Culture of Japan.
References
- Jones EY, Fugger L, Strominger JL, Siebold C. MHC class II proteins and disease: a structural perspective. Nat Rev Immunol. 2006;4:271–282. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- Nicholson MJ, Hahn M, Wucherpfennig KW. Unusual features of self-peptide/MHC binding by autoimmune T cell receptors. Immunity. 2005;23:351–360. doi: 10.1016/j.immuni.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;7:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- Müller-Hilke B, Mitchison NA. The role of HLA promoters in autoimmunity. Curr Pharm Des. 2006;12:3743–3752. doi: 10.2174/138161206778559759. [DOI] [PubMed] [Google Scholar]
- Mandik-Nayak L, Allen PM. Initiation of an autoimmune response: insights from a transgenic model of rheumatoid arthritis. Immunol Res. 2005;32:5–13. doi: 10.1385/IR:32:1-3:005. [DOI] [PubMed] [Google Scholar]
- Nepom GT, Kwok WW. Molecular basis for HLA-DQ associations with IDDM. Diabetes. 1998;47:1177–1184. doi: 10.2337/diab.47.8.1177. [DOI] [PubMed] [Google Scholar]
- Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007;19:44–48. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- Stratmann T, Apostolopoulos V, Mallet-Designe V, Corper AL, Scott CA, Wilson IA, Kang AS, Teyton L. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J Immunol. 2000;165:3214–3225. doi: 10.4049/jimmunol.165.6.3214. [DOI] [PubMed] [Google Scholar]
- van der Helm-van Mil AH, Wesoly JZ, Huizinga TW. Understanding the genetic contribution to rheumatoid arthritis. Curr Opin Rheumatol. 2005;17:299–304. doi: 10.1097/01.bor.0000160780.13012.be. [DOI] [PubMed] [Google Scholar]
- Fox RI, Stem M, Michelson P. Update in Sjogren’s syndrome. Curr Opin Rheumatol. 2000;12:391–398. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Loureiro J, Ploegh HL. Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions. Adv Immunol. 2006;92:225–305. doi: 10.1016/S0065-2776(06)92006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei M, Lamb JR, Bottazzo G. F. Feldmann M Epithelial cell expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984;312:639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- He XL, Radu C, Sidney J, Sette A, Ward ES, Garcia KC. Structural snapshot of aberrant antigen presentation linked to autoimmunity: the immunodominant epitope of MBP complexed with I-Au. Immunity. 2002;17:83–94. doi: 10.1016/s1074-7613(02)00340-0. [DOI] [PubMed] [Google Scholar]
- Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–676. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–289. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- Friese MA, Jones EY, Fugger L. MHC II molecules in inflammatory diseases: interplay of qualities and quantities. Trends Immunol. 2005;26:559–561. doi: 10.1016/j.it.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chang CH, Gourley TS, Sisk TJ. Function and regulation of class II transactivator in the immune system. Immunol Res. 2002;25:131–142. doi: 10.1385/IR:25:2:131. [DOI] [PubMed] [Google Scholar]
- Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109 Suppl:S21–S33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Wright KL, Ting JP. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 2006;27:405–412. doi: 10.1016/j.it.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, Leite-de-Moraes M, Dy M, Arnal JF, Bayard F, Herbelin A. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-γ production by invariant natural killer T cells. Blood. 2005;105:2415–2420. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
- Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023–6029. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren’s syndrome through Fas-mediated apoptosis. Am J Pathol. 1999;155:173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru N, Arakaki R, Watanabe M, Kobayashi M, Miyazaki K, Hayashi Y. Development of autoimmune exocrinopathy resembling Sjogren’s syndrome in estrogen deficient mice of healthy background. Am J Pathol. 2003;163:1481–1490. doi: 10.1016/S0002-9440(10)63505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottenberg J-E, Cagnard N, Lucchesi C, Letourneur F, Mituou S, Lazure T, Jacques S, Ba N, Ittah M, Lwpajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. 2006. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci USA. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- Harton JA, Ting JP. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol Cell Biol. 2000;20:6185–6194. doi: 10.1128/mcb.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri G, Deffrennes V, Prod'homme T, Vedrenne J, Baton F, Cortes C, Fischer A, Bono MR, Lisowska-Grospierre B, Charron D, Alcaïde-Loridan C. Isoforms of the class II transactivator protein. Int Immunol. 2002;14:839–48. doi: 10.1093/intimm/dxf060. [DOI] [PubMed] [Google Scholar]
- Piskurich JF, Linhoff MW, Wang Y, Ting JP. Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol Cell Biol. 1999;19:431–440. doi: 10.1128/mcb.19.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouker KB, Skaar TC, Fernandez DR, O'Brien KA, Riggins RB, Cao D, Clarke R. Interferon regulatory factor-1 mediates the proapoptotic but not cell cycle arrest effects of the steroidal antiestrogen ICI 182,780 (Faslodex, Fulvestrant). Cancer Res. 2004;64:4030–4039. doi: 10.1158/0008-5472.CAN-03-3602. [DOI] [PubMed] [Google Scholar]
- Zika E, Ting JP. Epigenetic control of MHC-II: interplay between CIITA and histone-modifying enzymes. Curr Opin Immunol. 2005;17:58–64. doi: 10.1016/j.coi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmu Rev. 2007;6:537–542. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Porola P, Laine M, Virkki L, Poduval P, Konttinen YT. The influence of sex steroids on Sjogren’s syndrome. Ann NY Acad Sci. 2007;1108:426–432. doi: 10.1196/annals.1422.045. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Ota S, Sekine C, Tada T, Otsuka T, Okamoto T, Sonderstrup G, Peterlin BM. Aberrant MHC class II expression in mouse joints leads to arthritis with extraarticular manifestations similar to rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:14465–14470. doi: 10.1073/pnas.0606450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Kimura M, Tzou SC, Chen YC, Suzuki K, Rose NR, Catureqli P. Expression of class II major histocompatibility complex molecules on thyrocytes does not cause spontaneous thyroiditis but mildly increases its severity after immunization. Endocrinol. 2005;146:1154–1162. doi: 10.1210/en.2004-1165. [DOI] [PubMed] [Google Scholar]
- Panek RB, Lee YJ, Itoh-Lindstrom Y, Ting JP, Benveniste EN. Characterization of astrocyte nuclear proteins involved in IFN-γ- and TNF-α-mediated class II MHC gene expression. J Immunol. 1994;153:4555–4564. [PubMed] [Google Scholar]
- Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol. 1998;161:5959–5966. [PubMed] [Google Scholar]
- Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994;152:2613–2623. [PubMed] [Google Scholar]
- Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]