Abstract
The morbidity burden associated with anti-neutrophil cytoplasmic autoantibody-associated vasculitis is increasing, and many novel biological therapies are now entering the drug development pipeline. There is thus an urgent need to develop a representative animal model to facilitate testing of these agents. We previously examined the effect of antineutrophil cytoplasmic autoantibody on leukocyte-endothelial interactions in WKY rats via immunization with human myeloperoxidase. We now seek to extend this model so that all animals reliably develop crescentic glomerulonephritis and lung hemorrhage. We also wish to investigate whether there is a genetic contribution to vasculitis development in this rat strain. Using escalating doses of human myeloperoxidase, we found that a dose of 1600 μg/kg induced pauci-immune crescentic glomerulonephritis and lung hemorrhage in all immunized animals. We also found that the addition of pertussis toxin and killed Mycobacterium tuberculosis to the adjuvant when immunizing with 400 μg/kg of myeloperoxidase resulted in crescentic glomerulonephritis and lung hemorrhage in all animals. However, when Lewis, Wistar Furth, or Brown Norway rats were immunized using a similar protocol, no animals developed hematuria or glomerulonephritis, despite having identical levels of anti-human myeloperoxidase antibodies. We conclude that, by adjusting the immunization regimen, all WKY rats immunized with myeloperoxidase develop experimental autoimmune vasculitis, thus facilitating future therapeutic studies. The resistance of Lewis rats to experimental autoimmune vasculitis provides a genetic basis for future studies of anti-myeloperoxidase antibody-associated vasculitis.
Small vessel systemic vasculitis is strongly associated with the presence of autoantibodies against constituents of neutrophil cytoplasmic granules (ANCAs).1,2 The two autoantigens targeted most frequently in this devastating condition are proteinase-33 and myeloperoxidase (MPO).4,5 Patients so affected may develop inflammatory necrosis of many organs, but it is pauci-immune crescentic glomerulonephritis and pulmonary hemorrhage that cause the greatest morbidity and mortality.6
There is now convincing evidence that antibodies directed against MPO are sufficient to induce vasculitis in a murine model.7 This elegant model, induced by taking high-affinity anti-MPO antibodies raised in MPO−/− mice and infusing them into naïve recipient mice, has facilitated study of the role of neutrophils,8 tumor necrosis factor-α,9 and complement10 in the pathogenesis of vasculitis. This field of research has progressed dramatically since this model was described. However, because the antibodies are raised in an animal that has never been exposed to the antigen before, the disease cannot be considered representative of autoimmune vasculitis. Therefore, although this model continues to illuminate the study of vasculitis pathogenesis, additional approaches are necessary to test the efficacy of novel therapies.
In 1993, Brouwer and colleagues11 induced crescentic glomerulonephritis in Brown Norway rats by infusing a lysosomal extract directly into the renal artery of animals immunized with human MPO. Although this model was hampered by the presence of glomerular immune deposits (the human disease being pauci-immune), it sparked a number of attempts to replicate human autoimmune vasculitis in rodents (summarized in Supplemental Figure 1, see http://ajp.amjpathol.org).12,13,14,15,16 We have previously built on this work by inducing anti-MPO antibodies in WKY rats by immunization with 400 μg/kg of human MPO.17 This inbred rat strain is known to be very susceptible to the induction of other forms of glomerulonephritis, such as experimental autoimmune glomerulonephritis.18 We demonstrated that the induced anti-MPO antibodies cross-reacted with rat neutrophils and were capable of augmenting leukocyte-endothelial interaction and inducing microvascular injury in vivo. We also found that these animals exhibited features of mild pauci-immune vasculitis, which was indistinguishable pathologically from human vasculitis, and described this as experimental autoimmune vasculitis (EAV). This model was subsequently used to investigate the efficacy of anti-tumor necrosis factor (TNF)-α antibodies in EAV.19
However, it has become evident that future therapeutic studies in EAV will require a more robust and reproducible disease phenotype. The current model is hampered by the low glomerular crescent fraction (average of 4 to 6%) and variable disease severity (only 50 to 60% of animals develop crescentic glomerulonephritis, with some immunized rats having normal kidney histology). We hypothesized that this variability occurred because the MPO dose used previously was on the steep component of the dose-response curve. Thus, we investigated whether increasing the dose or altering the adjuvant could induce disease in all treated animals. Additionally, we hypothesized that the WKY rat strain was particularly susceptible to EAV, thus potentially providing a basis for future genetic studies.
Materials and Methods
Animals
We obtained WKY/NCrlBR rats from Charles River (Margate, UK). In addition, we obtained Lewis, Sprague-Dawley, and Brown Norway rats from Harlan Olac (Bicestor, Oxford, UK), and Wistar Furth rats from Harlan (Indianapolis, IN). Rats were housed in cages of three to five in non specific pathogen-free conditions, with food and water available ad libitum. All animal studies were performed according to the directives of the UK Home Office Animals (Scientific Procedures) Act, UK (1986).
Immunization Regimen
Purified human MPO (Calbiochem, Merck Biosciences, Nottingham, UK) was reconstituted in sterile water for injection (500, 1000, and 2000 μg/ml for immunization of rats with 400, 800, and 1600 μg/kg, respectively) and mixed with an equal volume of complete Freund’s adjuvant (CFA) (Sigma-Aldrich, Gillingham, UK), with or without addition of killed Mycobacterium tuberculosis (4 mg/ml final concentration). Control animals received human serum albumin (HSA, 400 μg/kg) in an equal volume of CFA. In selected experiments, MPO- and HSA-immunized animals also received 400 to 800 ng of pertussis toxin (MP Biomedicals, Solon, OH) or vehicle intraperitoneally on days 0 and 2. In experiments examining alteration of the adjuvant constituents, the doses of MPO and HSA were fixed at 400 μg/kg.
Quantification of Anti-MPO Antibodies
Enzyme-Linked Immunosorbent Assay (ELISA)
Autoantibody levels in immunized rats were measured as described previously.17 Briefly, we coated 96-well plates overnight with hMPO (2 μg/ml) in carbonate buffer (pH 9.6). After washing, the serum samples (1:100) were incubated with hMPO for 60 minutes at 37°C, washed three times, and incubated with anti-rat or human IgG-alkaline phosphatase conjugate [in phosphate-buffered saline (PBS)/bovine serum albumin (BSA) 1%/0.1%Tween] for 45 minutes at 37°C. Binding was detected with p-NPP (Sigma) and optical density was quantified at 405 nm. For the purpose of determining anti-hMPO antibody titer, we performed log dilutions of serum down to 1:10,000 and defined the titer as that dilution that resulted in a drop of 50% in O.D. from that obtained with saturating antibody, with use of regression analysis to estimate the titer.
Inhibition ELISA
After coating a 96-well plate with hMPO, residual binding sites on the plastic were blocked with PBS/5% Marvel/1% Tween for 2 hours. We then mixed equal volumes of hMPO (in PBS/1% BSA) and serum from rats immunized with hMPO and control rats in triplicate to achieve final hMPO half-log dilutions from 0 to 30 μg/ml. We used a serum dilution from the steep component of the anti-hMPO dilution curve: 1:2000. After incubation for 2 hours, the remainder of the ELISA was performed as described above. To calculate the percent inhibition, we used the following formula20: 1 − [(S/MPO − C)/(S − C)] where, S = binding of sera from rats with EAV, S/MPO = binding of test sera after incubation with soluble hMPO, and C = binding of control sera.
Indirect Immunofluorescence
We incubated 50 μl of test serum from WKY rats immunized with MPO (EAV serum) and HSA (control serum) (all 1:20) with i) ethanol-fixed human neutrophil cytospins for 30 minutes; ii) paraformaldehyde-fixed, saponin (0.0001%)-permeabilized neutrophil suspensions for 20 minutes at 4°C; or iii) ethanol-fixed WKY rat mixed peritoneal exudate cytospins for 30 minutes. In experiments using rat leukocytes, to differentiate neutrophil from monocyte binding, we used an additional 30-minute incubation step with mouse anti-ED1 antibody or isotype control (1:500; Abcam, Cambridge, UK). After washing in PBS, we incubated with secondary antibody [Alexa-568/488-conjugated anti-rat IgG (1:50)] for 30 minutes. In experiments using anti-ED1 staining, the cells were also incubated with Alexa-633-conjugated anti-mouse IgG (1:50). After washing, images were captured with a laser-scanning confocal microscope (LSM5 Pascal; Zeiss, Jena, Germany).
Western Blot
Mixed leukocytes were prepared from WKY peripheral blood, lysed, and run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions. The purified hMPO (50 μg/ml) used to induce EAV and lysates of human neutrophils purified over percoll were used as positive controls. Samples were transferred to nitrocellulose membranes and incubated with primary antibody (IgG preparation from rats immunized with 1600 μg/kg MPO, 1 mg/ml in PBS/BSA 1%/Tween), followed by incubation with anti-IgG alkaline phosphatase conjugate, with all incubations being for 60 minutes. We detected binding using BCIP/NBT alkaline phosphatase substrate.
Assessment of Disease Phenotype
In all immunized animals, we collected the following data:
i) Hematuria and albuminuria. Urine was collected for 24 hours using metabolic cages and hematuria was assessed by dipstick. We measured urinary albumin excretion rate by Nephrat ELISA (Exocell, Philadelphia, PA).
ii) Creatinine was measured by the modified Jaffe reaction in an autoanalyzer.
iii) Urinary and serum TNF-α and monocyte chemoattractant protein-1 (MCP-1) were measured by ELISA according to the manufacturer’s (R&D, Abingdon, UK) instructions. For this purpose, 2-hour urinary collections were performed and the urine was snap-frozen in liquid nitrogen, with storage at −80°C until analysis.
iv) Renal glomerular histology and immunofluorescence. We used hematoxylin and eosin (H&E)- and periodic acid Schiff-stained sections to quantify focal proliferative glomerulonephritis and crescentic glomerulonephritis (as defined by the presence of at least two cell layers of proliferation in Bowman’s space). All histopathology scoring was performed without knowledge of the experimental conditions. Glomeruli classified as demonstrating focal proliferative glomerulonephritis often, but not always, had evidence of fibrinoid necrosis. The presence of a crescent superseded other glomerular changes; ie, if both were present, the glomerulus was scored as crescentic. In selected cases across all dose ranges we also quantified renal IgG deposition using immunofluorescence microscopy as described previously.17 The degree of fluorescence intensity in these sections was analyzed using Image-Pro plus image analysis software (MediaCybernetics, Bethesda, MD). To exclude the possibility that renal injury occurred secondary to anti-human MPO antibodies binding to MPO deposited in the glomeruli, we performed immunohistochemical staining of renal and splenic tissue using polyclonal rabbit anti-human MPO (1:100; DAKO, Glostrup, Denmark) or control IgG. Binding was detected with peroxidase and diaminobenzidine (EnVision, DAKO). Renal sections from a patient with vasculitic crescentic glomerulonephritis were used for comparison.
v) Tubulointerstitial nephritis (TIN). In addition to glomerular changes, it was appreciated in early studies of EAV that TIN was a frequent finding. We quantified this with a global visual analogue score (0 to 3): 0 = no TIN; 1 = a single focus of TIN; 2 = <25% of tubulointerstitium involved; and 3 = >25% of tubulointerstitium involved.
vi) Severity of lung hemorrhage. To accurately assess lung histology, it is necessary to intubate the trachea to ensure full lung inflation at the time of fixation.21 We did not do this, so it was not possible to accurately quantify lung vasculitis, beyond general comments about the presence or absence of intra-alveolar hemorrhage and perivascular leukocyte cuffing. We did, however, find that the lungs of many treated rats displayed petechiae over their surface at the time of sacrifice. We quantified this using a lung petechiae score (0 to 4). Because the right lung was clearly visible without manipulation at the time of thoracotomy, we used this lung for quantification purposes: 0 = no hemorrhage; 1 = a single lesion; 2 = 2 to 5 lesions; 3 = 6 to 12 lesions; and 4 = more than 12 lesions/massive macroscopic lung hemorrhage.
Statistical Analysis
Changes in antibody levels across various hMPO doses were compared using two-way analysis of variance. Variation in hematuria, albuminuria, glomerular crescent score, lung hemorrhage, and TIN were assessed using the Kruskal-Wallis test, with use of Dunn’s posthoc test to compare individual groups. Creatinine clearance was compared using analysis of variance, with posthoc testing for linear trend with increasing dose of hMPO.
Results
Antibody Response of WKY Rats Immunized with hMPO: Time Course and Effect of hMPO Dose
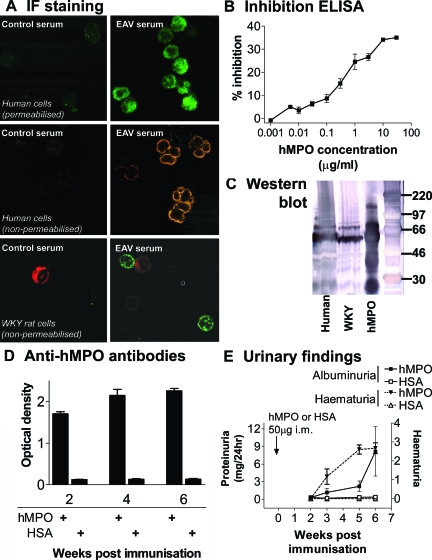
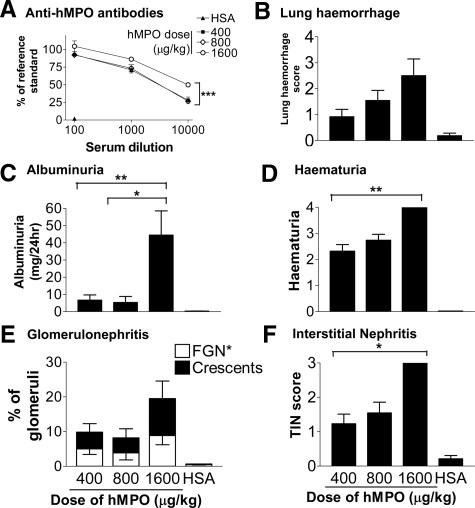
All rats immunized with hMPO developed anti-hMPO antibodies by day 14 after immunization (Figure 1). Using indirect immunofluorescence on fixed human neutrophils, sera from MPO-immunized rats exhibited a typical perinuclear pattern of binding (Figure 1A) that was indistinguishable from the pattern produced by serum from patients with MPO-ANCA-associated systemic vasculitis. As shown previously, these sera cross-reacted with WKY rat neutrophils as demonstrated by indirect immunofluorescence on WKY neutrophils, Western blot (Figure 1C), and by flow cytometry (data not shown).17 The antibodies bound to a 55-kDa band consistent with the heavy chain of MPO. By 6 weeks, hMPO-immunized rats had anti-hMPO titers of >1:1600 by ELISA. To ensure that the results obtained with ELISA were indicative of specific anti-hMPO antibodies, we performed inhibition ELISA using escalating concentrations of soluble hMPO. This confirmed that the binding of serum to immobilized hMPO was specifically inhibited in a log-linear manner (Figure 1B). Rats immunized with HSA were negative for anti-hMPO antibodies (Figure 1D). Anti-hMPO antibody levels were significantly higher in rats immunized with 1600 μg/kg of hMPO (Figure 2A). Median titers were 1:3500 (IQR 1900 to 13000), 1:4000 (IQR 2800 to 7500), and 1:15,900 (range, 4400 to 33,000) in 400, 800, and 1600 μg/kg groups, respectively.
Figure 1.
Antibody response and urinary changes in hMPO-immunized rats. A: Binding of EAV serum to human and WKY neutrophils. Permeabilized, paraformaldehyde-fixed human neutrophil suspensions (top) were incubated with serum (1:20) from rats immunized with HSA or hMPO and binding was detected with Alexa-488 anti-rat IgG. Middle: Binding patterns on human neutrophil cytospins fixed in 95% ethanol when incubated with serum (1:20) from rats immunized with saline and hMPO. Binding was detected with Alexa-568 anti-rat IgG. Bottom: Double-staining experiments on rat leukocyte cytospins using serum (1:20, detected with Alexa-488 anti-rat IgG, green) from rats immunized with HSA and hMPO (1600 μg/kg), and ED1 antibody against monocytes (detected with Alexa-633 anti-mouse IgG, red). All images were captured with a confocal microscope. Representative of three separate experiments. B: Specific inhibition of binding of serum from rats with EAV to hMPO immobilized on plastic by soluble hMPO. Serum (1:2000) was incubated with hMPO in the presence of increasing concentrations of soluble hMPO. The data points represent the mean ± range of two experiments. C: IgG from rats with EAV reacts with rat neutrophil lysates. Lysates of peripheral blood leukocytes were prepared from human (lane 1) and WKY (lane 2) blood and separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions. Purified hMPO (as used to induce EAV) was also run as a positive control (lane 3). The blot was probed with Ig prepared from animals immunized with hMPO (EAV IgG 1 mg/ml). Binding was detected using alkaline phosphatase conjugate. D: WKY rats immunized with hMPO develop high titers of anti-hMPO antibodies. Anti-hMPO antibodies in selected serum samples (1:100) collected at various time points after immunization with 400 μg/kg of hMPO or HSA were measured using ELISA. The bars represent the mean ± SEM optical density (n = 5 in each group). E: WKY rats immunized with hMPO develop hematuria and albuminuria throughout time. Hematuria was assessed semiquantitatively with a urinary dipstick. Urinary albumin excretion rate was measured with ELISA at various time points. The bars represent the mean ± SEM optical density (n = 5 in each group).
Figure 2.
Vasculitis phenotype 6 weeks after immunization with a dose range from 400 to 1600 μg/kg of hMPO or HSA in WKY rats. A: Anti-hMPO antibodies in serially diluted serum were assessed using ELISA. B: Lung hemorrhage was scored macroscopically at the time of sacrifice. Bars represent the mean ± SEM. C: Urinary albumin excretion rate was measured with ELISA. Bars represent the mean ± SEM. D: Hematuria was assessed semiquantitatively with a urinary dipstick. Bars represent the mean ± SEM. E: Glomerular changes (focal proliferative glomerulonephritis and crescent formation) were assessed blindly using H&E and periodic acid-Schiff-stained sections. Bars represent the mean ± SEM. F: Severity of TIN was assessed blindly using H&E-stained sections. Bars represent the mean ± SEM. For all data, n = 4 to 12 separate animals for each dose of hMPO. Statistically significant differences between groups are demonstrated with asterisks, *P < 0.05, **P < 0.01, ***P < 0.05.
Phenotype of WKY Rats Immunized with hMPO: Urinary Abnormality
All rats immunized with 400 μg/kg of hMPO developed hematuria by 6 weeks and six (50%) developed proteinuria (>1 mg/24 hours, Figure 1E). Mean albumin excretion rate was significantly greater in EAV rats (8.1 ± 4.2 mg versus 0.4 ± 0.05 mg in EAV and control rats, respectively, P < 0.005; Figure 1E). None of the control rats developed hematuria. It appeared that the biggest effect of dose on urinary findings came with doubling the hMPO dose from 800 to 1600 μg/kg (Figure 2, B–F), with macroscopic hematuria (grade 4) evident in all rats immunized with the higher dose by 6 weeks after immunization. Consistent with this, there was a large increase in albuminuria in this group (mean at 6 weeks: 44.5 ± 14.1 mg/24 hours). There was a trend toward a reduction in excretory renal function with increasing doses of hMPO, although creatinine clearance remained within the normal range for WKY rats in all groups. Mean creatinine clearance was 1.7 ± 0.1 ml/minute in HSA-immunized animals, and 1.4 ± 0.1, 1.4 ± 0.1, and 1.2 ± 0.2 in the 400, 800, and 1600 μg/kg groups, respectively (P = 0.09, P < 0.05 posttest for linear trend).
Phenotype of WKY Rats Immunized with hMPO: Renal Pathology
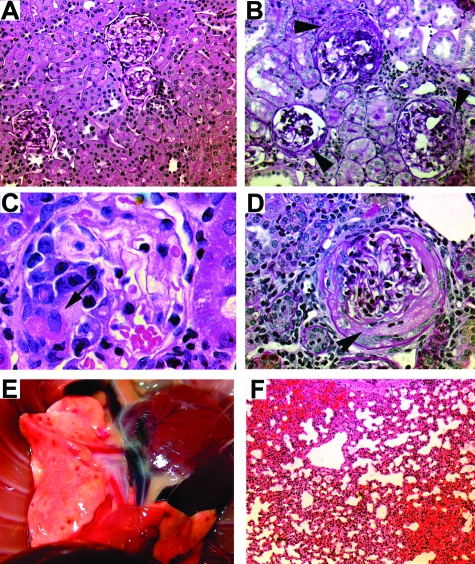
Immunization with hMPO induced focal proliferative glomerulonephritis with patchy fibrinoid necrosis and crescentic glomerulonephritis. The glomerular lesion was variable both between and within animals. The percentage of animals developing crescentic glomerulonephritis was 46%, 64%, and 100% in the groups receiving 400, 800, and 1600 μg/kg, respectively. In those animals developing crescents, the renal injury was focal (mean percentage of crescentic glomeruli 4.7 ± 2.4, 3.8 ± 2.4, and 10.5 ± 5.1% in groups receiving 400, 800, and 1600 μg/kg, respectively, Figure 2). There was minimal glomerular deposition of immunoglobulin by immunofluorescence at all doses used, as found previously and illustrated in Supplemental Figures 1 to 3, see http://ajp.amjpathol.org.17 No hMPO deposits were detectable in kidney sections; occasional positive staining cells were evident in the spleens of both HSA and hMPO-immunized animals (Supplemental Figure 4, see http://ajp.amjpathol.org). All animals immunized with 1600 μg/kg of hMPO developed severe interstitial nephritis (TIN, Figure 2). The prominent hematuria on dipstick was reflected in the presence of numerous red cell casts, often spatially associated with injured glomeruli. No crescents were seen in control rats immunized with HSA.
Phenotype of WKY Rats Immunized with hMPO: Lung Pathology
At the time of sacrifice, 70%, 73%, and 100% of rats immunized with 400, 800, and 1600 μg/kg of hMPO, respectively, had petechiae over the surface of the lungs (Figure 3). These were bright red and macular and associated with histological evidence of alveolar hemorrhage. The macroscopic lung hemorrhage score was significantly higher in hMPO-immunized rats when compared with HSA-immunized control rats (mean 1.0 ± 0.3, 1.6 ± 0.4, 2.5 ± 0.6, and 0.4 ± 0.2 in 400 μg/kg, 800 μg/kg, 1600 μg/kg, and HSA groups, respectively; Figure 2).
Figure 3.
Histological changes 6 weeks after immunization with a dose range from 400 to 1600 μg/kg of hMPO or HSA in WKY rats. A: Normal appearing glomeruli, tubules, and interstitium of rats immunized with 400 μg/kg of HSA. Periodic acid-Schiff. B: Crescentic glomerulonephritis (arrowheads) in a rat immunized with 400 μg/kg of hMPO, periodic acid-Schiff. C: Fibrinoid necrosis (arrow) in a rat immunized with 800 μg/kg of hMPO, H&E. D: Circumferential crescent formation (arrowhead) in a rat immunized with 1600 μg/kg of hMPO, periodic acid-Schiff. E: Macroscopic appearance of lung petechiae in a rat immunized with 1600 μg/kg of hMPO. F: Alveolar hemorrhage in a rat immunized with 800 μg/kg of hMPO, H&E. Original magnifications: ×10 (A); ×20 (B, F); ×100 (C); ×40 (D).
Phenotype of WKY Rats Immunized with hMPO: Monocyte Chemoattractant Protein-1 (MCP-1) and Tumor Necrosis Factor (TNF)-α
Levels of TNF-α were low in both serum and urine, with no difference between hMPO (400 μg/kg) and HSA-immunized animals. Mean serum TNF-α concentration was 3.3 ± 0.6 pg/ml and 3.7 ± 0.7 pg/ml in EAV and control rats, respectively. Levels in the urine were in many cases below the detection limit for the assay (0.5 pg/ml), with no difference between the experimental groups (2.9 ± 1.0 pg/mmol and 2.6 ± 1.0 pg/mmol creatinine in EAV and control rats, respectively). On the other hand, urine MCP-1 levels were relatively high, although there were again no differences between hMPO- and HSA-immunized rats (22.1 ± 3.0 ng/mmol and 25.3 ± 8.0 ng/mmol creatinine in EAV and control rats, respectively).
Addition of Pertussis Toxin and Customization of CFA
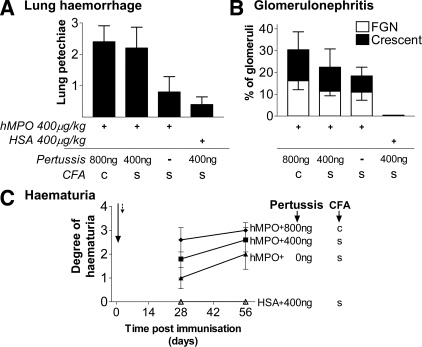
Addition of the adjuvant pertussis toxin 200 ng i.p. to the 400 μg/kg MPO in CFA immunization regimen on days 0 and 2 increased hematuria nonsignificantly, and had no effect on albuminuria (Figure 4C). Increasing the pertussis toxin dose to two doses of 400 ng, combined with modification of CFA by addition of killed M. tuberculosis, further increased hematuria to the extent that hMPO (400 μg/kg)-immunized animals had more extensive hematuria at day 28 than animals immunized with the standard protocol at day 56. No rats immunized with HSA/CFA + pertussis developed hematuria or albuminuria. The addition of pertussis toxin to the immunization regimen increased lung hemorrhage (Figure 4A). All animals immunized with 400 μg/kg of hMPO (in modified CFA) + 800 ng of pertussis toxin had lung hemorrhage at the time of sacrifice.
Figure 4.
Renal and pulmonary effects of pertussis toxin and customized CFA in EAV. WKY rats were immunized with 400 μg/kg of hMPO or HSA in either standard (s) or customized (c) CFA. In addition, rats received 400 to 800 ng of pertussis toxin or vehicle intraperitoneally on days 0 (arrow) and 2 (dotted arrow). A: Lung hemorrhage was scored blindly at the time of sacrifice on day 56. Bars represent the mean ± SEM. B: Glomerular changes 6 weeks after immunization were assessed blindly using H&E- and periodic acid-Schiff-stained sections. One hundred percent of animals immunized with 400 μg/kg of hMPO in customized CFA with 800 ng of pertussis toxin developed crescentic GN. Bars represent the mean ± SEM. C: Rats were immunized as in A and hematuria was assessed at days 28 and 56. Each point represents the mean ± SEM. For all data n = 5 in each group.
The use of pertussis toxin and modified CFA had moderate effects on the severity of glomerulonephritis (Figure 4B). Specifically, addition of 800 ng of pertussis toxin and modification of CFA to the standard immunization regimen (400 μg/kg of MPO) resulted in a more consistent disease phenotype, with all animals in this group developing crescentic nephritis. Mean percentage of glomeruli with crescents in the group receiving high-dose pertussis toxin and modified CFA was 14.0 ± 8.2%. Similarly, all rats so immunized had evidence of TIN [mean TIN score 0, 1.8 ± 0.6, 1.8 ± 0.4, and 2.4 ± 0.4 in rats immunized with HSA + pertussis, hMPO + vehicle, hMPO + pertussis (400 ng), and hMPO + pertussis (800 ng) in modified CFA, respectively), P = NS].
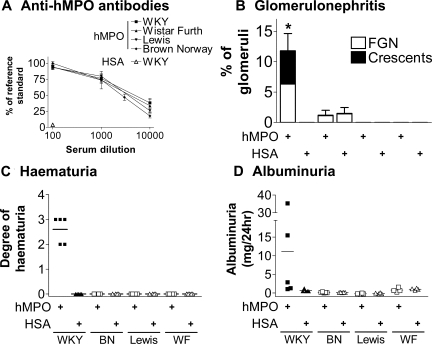
Effect of Rat Strain on EAV Phenotype
In response to immunization with 400 μg/kg of hMPO, WKY, BN, Lewis, and WF rats all developed high levels of anti-hMPO antibodies, as measured by ELISA (Figure 5A). There were no significant differences between the four strains with respect to antibody titer. None of the strains developed anti-hMPO antibodies in response to HSA immunization. By 6 weeks after immunization, all WKY rats developed hematuria and half developed mild albuminuria (Figure 5, C and D), whereas no urinary abnormalities were seen in the other rat strains. Consistent with this, only WKY rats developed crescentic nephritis. When analyzed in a blinded manner, some BN rats were scored as having focal proliferative glomerular changes (Figure 5B). This finding was the same in hMPO and HSA groups and reflects the relatively hypercellular nature of BN glomeruli.
Figure 5.
Phenotype 6 weeks after immunization with 400 μg/kg of hMPO or HSA in WKY, Brown Norway (BN), Lewis, and Wistar Furth (WF) rats. A: Anti-hMPO antibodies in serially diluted serum were measured using ELISA 6 weeks after immunization with HSA or hMPO. Each point indicates the mean ± SEM for each rat strain. B: Glomerular changes 6 weeks after immunization were assessed blindly using H&E- and periodic acid-Schiff-stained sections. Bars represent the mean ± SEM, *P < 0.05. C: Hematuria was assessed semiquantitatively with a urinary dipstick. Each data point represents an individual animal and the bars indicate the mean. D: Urinary albumin excretion rate was assessed with ELISA. Each data point represents an individual animal and the bars indicate the median. n = 4 to 5 rats in each group.
Discussion
Using a model of ANCA induction by immunization with hMPO (previously used to investigate the impact of this autoantibody on leukocyte-endothelial interaction), we have investigated whether increasing antigen dose or modifying immunization adjuvant can lead to a more robust representation of human vasculitis. We found that increasing hMPO antigen dose to 1600 μg/kg, or adding a combination of pertussis toxin and killed M. tuberculosis to the adjuvant, results in crescentic nephritis and lung hemorrhage in all immunized rats. Similar to human vasculitis, the pauci-immune renal disease was focal, with areas of normal kidney adjacent to severely damaged areas, and the overall fraction of glomeruli with crescents being 10 to 14%. We also investigated whether other inbred rat strains were susceptible to induction of vasculitis by immunization with hMPO. The finding of complete resistance to vasculitis in the non-WKY strains tested (despite all strains developing high titers of anti-hMPO antibodies) paves the way for genetic studies to look for vasculitis susceptibility genes.
A dose of 10 μg (equivalent to ∼80 μg/kg) of hMPO given subcutaneously to WKY rats induced anti-MPO antibodies but no vasculitis in previous studies.22 We have found that a much greater dose is required to induce vasculitis reliably. The renal disease in this model is characterized primarily by hematuria (as in human systemic vasculitis), although more severely affected animals developed moderate degrees of albuminuria. We were unable to demonstrate a consistent effect of vasculitis severity on urinary MCP-1 excretion, a biomarker that is under investigation in human vasculitis.23 This may have occurred because of the marked generalized effect of Freund’s adjuvant on macrophage function, this making it impossible to differentiate MCP1 production between hMPO- and HSA-immunized animals.
The greatest effect on disease phenotype of increasing antigen dose occurred between 800 and 1600 μg/kg. That lower doses produced a very variable response suggests that these doses are on the lower part of antigen dose-response curve, and that 1600 μg/kg is near the top part of the curve. It is conceivable that further increases of dose may induce more severe disease, to the extent that excretory renal function is impaired (as seen in many cases of human vasculitis). Indeed, an analogous rodent model of crescentic glomerulonephritis, experimental autoimmune glomerulonephritis, a model of human anti-glomerular basement membrane antibody disease, requires the use of 5000 μg/kg of solubilized rat glomerular basement membrane24 or 800 μg/kg of purified Goodpasture’s antigen (NC1 domain of the α3 chain of type IV collagen).25 However, higher doses of hMPO would require a renewable source of recombinant hMPO because purchase of commercial purified hMPO becomes prohibitively expensive at greater than a dose of 1600 μg/kg.
We found that, among four rat strains tested, only the WKY rat was susceptible to the induction of systemic vasculitis after immunization with hMPO in CFA. This is the first experimental demonstration of a definite genetic contribution to autoimmune anti-MPO antibody-associated vasculitis. Of particular note is the finding that resistance to disease occurred despite the induction of similar titers of ANCA. Lewis and WKY rats share the same MHC haplotype (RT1l), thus indicating that the susceptibility to EAV involves non-MHC-linked genes. Although it is possible that there was a qualitative difference between the antibody type induced in the different rat strains to account for the absence of disease despite similar titers of anti-MPO antibody (eg, variation in IgG subtype or a greater fraction of antibody reactive against rat MPO in WKY rats), we believe it is more likely that the essential functional difference between the rat strains is further downstream in the effector arm of the immune response. For example, it has been found that WKY macrophages are much more effective at killing antibody-coated mesangial cells in vitro.26 Two other rodent models of crescentic nephritis, experimental autoimmune glomerulonephritis and nephrotoxic nephritis (NTN), are induced by immunization with glomerular basement membrane antigens and injection of nephrotoxic serum, respectively.24,27,28 Studies from our group into the genetic contribution to these models have focused on the finding that WKY rats are susceptible to disease induction, whereas Lewis rats are resistant.29,30 As with our attempts at inducing EAV in the Lewis strain, Lewis rats injected with glomerular basement membrane develop high titers of antibody but no glomerulonephritis. Genome-wide linkage analyses using various breeding strategies have shown a significant area of linkage to glomerulonephritis susceptibility on chromosomes 13 and 16 in NTN, and we have demonstrated that Fcγ-receptor copy number underlies some of this variation in susceptibility to NTN.26 Lewis rats are also resistant to the induction of EAV, thus providing the basis for similar genetic studies. Through the use of Lewis/WKY congenic strains, it should be feasible to investigate in more depth the exact contribution of various target loci on the WKY genome to the development of EAV.
Adjuvants are crucial in animal models of autoimmunity in that most autoantigens are insufficiently potent to induce an adequate immune response on their own. Pertussis toxin is a potent immune stimulator that is used in the induction of experimental autoimmune encephalitis,31 and the M. tuberculosis fraction of CFA, primarily by activating the innate immune system, is essential for the induction of a cell-mediated immune response.32 Use of a regimen using pertussis (400 ng on days 0 and 2), in addition to making up the hMPO antigen in CFA to which further M. tuberculosis had been added, had the effect of inducing crescents in all animals. The severity of glomerulonephritis induced by 400 μg/kg of hMPO using this adjuvant was similar in magnitude to that seen after immunization with 1600 μg/kg of hMPO in standard CFA. Thus, this may be a more economical approach to inducing reliable disease for therapeutic studies than progressive increases in antigen dose. It is also possible that combining higher antigen doses with modification of the adjuvant may further increase disease severity.
As mortality rates have fallen with increased use of cyclophosphamide-based treatment regimens, the prevalence of ANCA-associated vasculitis in the community (with its associated morbidity burden) has increased. Much of this disease-associated morbidity is related to treatment toxicity. Therefore, it is encouraging that the last 5 years has seen a number of promising novel biological agents for treatment of this disease enter the drug development pipeline. The need for a reliable representative animal model of human autoimmune small vessel systemic vasculitis has never been more acute. Although the revised EAV model described here is not characterized by the progressive renal failure seen in human ANCA-associated vasculitis, we have demonstrated that it is possible to induce crescentic glomerulonephritis and lung hemorrhage in all WKY rats immunized with hMPO. This is of critical importance to future therapeutic studies because it allows one to markedly reduce the number of animals required to demonstrate a statistical effect of a given treatment. Our knowledge of the unique pathogenic mechanisms operating in ANCA-associated vasculitis has expanded greatly in the past 5 years. We are now in a position to begin developing novel biological agents designed to inhibit specific components of this pathogenic pathway. We believe that the revised EAV model will play an important role in such drug development.
Supplementary Material
Footnotes
Address reprint requests to Prof. Charles Pusey, Renal Section, Division of Medicine, Imperial College London, Hammersmith Campus, London W12 0NN, UK. E-mail: c.pusey@imperial.ac.uk.
Supported by the Wellcome Trust (to M.A.L.), Kidney Research UK (to L.S.) and British Heart Foundation (D.H.).
This work has been presented in part at the American Society of Nephrology annual meeting of 2005.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.A.L.: Division of Immunity and Infection, University of Birmingham, Edgbaston, UK.
References
- Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed) 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1:425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener’s granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–1893. [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Hoidal JR, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–2264. [PubMed] [Google Scholar]
- Little MA, Nazar L, Farrington K. Outcome in glomerulonephritis due to systemic small vessel vasculitis: effect of functional status and non-vasculitic co-morbidity. Nephrol Dial Transplant. 2004;19:356–364. doi: 10.1093/ndt/gfg551. [DOI] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JWC, Jennette JC, Heeringa P. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-{alpha}. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer E, Huitema MG, Klok PA, de Weerd H, Tervaert JW, Weening JJ, Kallenberg CG. Antimyeloperoxidase-associated proliferative glomerulonephritis: an animal model. J Exp Med. 1993;177:905–914. doi: 10.1084/jem.177.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjoh K, Kyogoku M, Good RA. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc Natl Acad Sci USA. 1993;90:3413–3417. doi: 10.1073/pnas.90.8.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa P, Brouwer E, Klok PA, Huitema MG, van den Born J, Weening JJ, Kallenberg CG. Autoantibodies to myeloperoxidase aggravate mild anti-glomerular-basement-membrane-mediated glomerular injury in the rat. Am J Pathol. 1996;149:1695–1706. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Shibata T, Sugisaki T. Aggravation of rat nephrotoxic serum nephritis by anti-myeloperoxidase antibodies. Kidney Int. 1995;47:454–463. doi: 10.1038/ki.1995.58. [DOI] [PubMed] [Google Scholar]
- Harper JM, Thiru S, Lockwood CM, Cooke A. Myeloperoxidase autoantibodies distinguish vasculitis mediated by anti-neutrophil cytoplasm antibodies from immune complex disease in MRL/Mp-lpr/lpr mice: a spontaneous model for human microscopic angiitis. Eur J Immunol. 1998;28:2217–2226. doi: 10.1002/(SICI)1521-4141(199807)28:07<2217::AID-IMMU2217>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mathieson PW, Thiru S, Oliveira DB. Mercuric chloride-treated brown Norway rats develop widespread tissue injury including necrotizing vasculitis. Lab Invest. 1992;67:121–129. [PubMed] [Google Scholar]
- Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Mavromatidis K, Cashman SJ, Evans DJ, Pusey CD. Experimental autoimmune glomerulonephritis (EAG) induced by homologous and heterologous glomerular basement membrane in two substrains of Wistar-Kyoto rat. Nephrol Dial Transplant. 1998;13:44–52. doi: 10.1093/ndt/13.1.44. [DOI] [PubMed] [Google Scholar]
- Little MA, Bhangal G, Smyth CL, Nakada MT, Cook HT, Nourshargh S, Pusey CD. Therapeutic effect of anti-TNF-alpha antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J Am Soc Nephrol. 2006;17:160–169. doi: 10.1681/ASN.2005060616. [DOI] [PubMed] [Google Scholar]
- Patry YC, Nachman PH, Audrain MA, Falk RJ, Meflah K, Esnault VL. Difference in antigenic determinant profiles between human and rat myeloperoxidase. Clin Exp Immunol. 2003;132:505–508. doi: 10.1046/j.1365-2249.2003.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidebach W, Viana VS, Leon EP, Bueno C, Leme AS, Arantes-Costa FM, Martins MA, Saldiva PH, Bonfa E. C-ANCA-positive IgG fraction from patients with Wegener’s granulomatosis induces lung vasculitis in rats. Clin Exp Immunol. 2002;129:54–60. doi: 10.1046/j.1365-2249.2002.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer E, Weening JJ, Klok PA, Huitema MG, Tervaert JW, Kallenberg CG. Induction of an humoral and cellular (auto) immune response to human and rat myeloperoxidase(MPO) in Brown-Norway(BN), Lewis and Wistar Kyoto(WKY) rat strains. Adv Exp Med Biol. 1993;336:139–142. doi: 10.1007/978-1-4757-9182-2_24. [DOI] [PubMed] [Google Scholar]
- Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, Cook HT, Kallenberg CG, Gaskin G, Levy JB, Pusey CD. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–2768. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Moss J, Duda MA, Smith J, Karkar AM, Macherla V, Shore I, Evans DJ, Woodrow DF, Pusey CD. The evolution of crescentic nephritis and alveolar haemorrhage following induction of autoimmunity to glomerular basement membrane in an experimental model of Goodpasture’s disease. J Pathol. 2003;200:118–129. doi: 10.1002/path.1336. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Prodromidi EI, Juggapah JK, Abbott DS, Holthaus KA, Kalluri R, Pusey CD. Nasal administration of recombinant rat {alpha}3(IV)NC1 prevents the development of experimental autoimmune glomerulonephritis in the WKY rat. J Am Soc Nephrol. 2005;16:1350–1359. doi: 10.1681/ASN.2004121026. [DOI] [PubMed] [Google Scholar]
- Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- Karkar AM, Tam FW, Steinkasserer A, Kurrle R, Langner K, Scallon BJ, Meager A, Rees AJ. Modulation of antibody-mediated glomerular injury in vivo by IL-1ra, soluble IL-1 receptor, and soluble TNF receptor. Kidney Int. 1995;48:1738–1746. doi: 10.1038/ki.1995.472. [DOI] [PubMed] [Google Scholar]
- Dean EG, Wilson GR, Li M, Edgtton KL, O'Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR. Experimental autoimmune Goodpasture’s disease: a pathogenetic role for both effector cells and antibody in injury. Kidney Int. 2005;67:566–575. doi: 10.1111/j.1523-1755.2005.67113.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Cook PR, Ryan JJ, Norsworthy PJ, Glazier AM, Duda MA, Evans DJ, Aitman TJ, Pusey CD. Segregation of experimental autoimmune glomerulonephritis as a complex genetic trait and exclusion of Col4a3 as a candidate gene. Exp Nephrol. 2002;10:402–407. doi: 10.1159/000065297. [DOI] [PubMed] [Google Scholar]
- Cook PR, Evans DJ, Reynolds J, Aitman TJ, Pusey C. Genetic analysis of experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2004;15:664A. Abstract SuPO601. [Google Scholar]
- Montero E, Nussbaum G, Kaye JF, Perez R, Lage A, Ben-Nun A, Cohen IR. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun. 2004;23:1–7. doi: 10.1016/j.jaut.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Schols D, De Wolf-Peeters C, Billiau A. Enhanced autoimmune arthritis in IFN-gamma receptor-deficient mice is conditioned by mycobacteria in Freund’s adjuvant and by increased expansion of Mac-1+ myeloid cells. J Immunol. 1999;163:3503–3510. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.