Abstract
Psoriasis is initiated and maintained through a multifaceted interplay between keratinocytes, blood vessels, gene expression, and the immune system. One previous psoriasis model demonstrated that overexpression of the angiopoietin receptor Tie2 in endothelial cells and keratinocytes led to the development of a psoriasiform phenotype; however, the etiological significance of overexpression in each cell type alone was unclear. We have now engineered two new mouse models whereby Tie2 expression is confined to either endothelial cells or keratinocytes. Both lines of mice have significant increases in dermal vasculature but only the KC-Tie2-overexpressing mice developed a cutaneous psoriasiform phenotype. These mice spontaneously developed characteristic hallmarks of human psoriasis, including extensive acanthosis, increases in dermal CD4+ T cells, infiltrating epidermal CD8+ T cells, dermal dendritic cells and macrophages, and increased expression of cytokines and chemokines associated with psoriasis, including interferon-γ, tumor necrosis factor-α, and interleukins 1α, 6, 12, 22, 23, and 17. Host-defense molecules, cathelicidin, β-defensin, and S100A8/A9, were also up-regulated in the hyperproliferative skin. All of the phenotypic traits were completely reversed without any scarring following repression of the transgene and were significantly improved following treatment with the anti-psoriasis systemic therapeutic, cyclosporin A. Therefore, confining Tie2 overexpression solely to keratinocytes results in a mouse model that meets the clinical, histological, immunophenotypic, biochemical, and pharmacological criteria required for an animal model of human psoriasis.
Psoriasis is a chronic inflammatory disease that affects ∼2% of the general population and is characterized by well demarcated erythematous plaques, keratinocyte (KC) hyperproliferation and differentiation, and increases in angiogenesis and inflammatory cell infiltrates. The initial triggers leading to psoriasis and the contribution of the several identified genetic loci associated with psoriasis remains unclear.
Animals models targeting overexpression of specific cytokines and growth factors associated with human psoriasis recapitulate some but not all aspects of human disease including epidermal hyperplasia, leukocyte infiltration, increased angiogenesis, and increased presence of inflammatory cytokines (reviewed in1,2). Due to the partial phenotype these models have been limited in their usefulness in providing insight into the etiology of psoriasis.
Overexpression of the pro-angiogenic, pro-inflammatory molecule vascular endothelial growth factor (VEGF) in KCs leads to significant increases in dermal angiogenesis, and the subsequent development of a psoriasis-like phenotype in two independent murine models.3,4 To initiate the psoriasis phenotype, one model required triggering an inflammatory response using oxazolone,4 the other model required either inducing a wound (eliciting a Koebner like response) or allowing the mouse to mature for 6 months, at which time a phenotype developed spontaneously.3 In both cases, the phenotypes were reversible following inhibition of VEGF signaling using antibodies directed against VEGF or the VEGF receptors. We previously reported that overexpression of the angiopoietin receptor Tie2 using a Tie2 promoter resulted in increased dermal angiogenesis and the development of a flakey erythematous skin phenotype that included the presence of CD3+ T cells.5 However, the use of the Tie2 promoter led to gene expression in not only endothelial cells (ECs) as was expected and previously confirmed,6 but also in KCs. Therefore it was unclear whether the KC- or EC-derived increase in Tie2 was critical for development of the psoriasiform phenotype. If EC-Tie2 overexpression was sufficient to induce the development of a psoriasiform phenotype, this would suggest a causative role for the dermal vasculature in the development of the disease.
To compartmentalize Tie2 overexpression to either ECs or KCs, we engineered two new lines of mice with Tie2 gene expression confined to either ECs, driven by the Tie1 promoter, or KCs, driven by the K5 promoter. EC-Tie2 mice failed to develop a psoriasis-like phenotype whereas KC-Tie2 mice developed a strong spontaneously occurring phenotype which was characteristically distinct from that previously described for the KC-EC-Tie2 overexpressing mouse.5,7 This mouse model bears a striking resemblance to human psoriasis meeting multiple criteria at the clinical, histological, biochemical, immunophenotypic, and pharmacological levels.
Materials and Methods
Transgenic Mice and Systemic Treatment Approaches
The KC-specific (K5-tTA) and EC-specific (Tie1-tTA) driver lines and the TetosTek/Tie2 responder line of mice, generated on the outbred CD1 strain, were previously engineered6,8,9 and were generously provided by Drs. Adam Glick (Pennsylvania State University, PA) and Daniel Dumont (Sunnybrook Women’s College Research Center, Toronto, Canada). Mating was completed between K5-tTa or Tie1-tTa and the TetOS-Tek lines. At weaning, DNA was extracted from ear punch tissues by incubating in 50 mmol/L NaOH for 1 hour at 95°C and neutralized with 1M Tris, pH 8. PCR was used for genotyping and was performed using primers as previously described.6,9 Littermates that inherited one or no transgenes served as experimental controls. Gene repression of the KC-Tie2 transgenic mice was accomplished by providing animals with 100 μg/ml doxycycline (Sigma-Aldrich, St. Louis, MO) with 5% sucrose in their drinking water. Doxycycline-containing water was changed at least twice weekly for a period of 4 weeks. CsA administration (80 mg/kg, Sigma-Aldrich) was accomplished via oral gavage three times per week for 4 weeks. For longitudinal experiments, before treatment was initiated, a 6 mm skin biopsy was taken from each mouse, half of which was stained with H&E, the other half used for immunohistochemistry. Following 4 weeks of treatment, mice were euthanized, and post-treatment tissues collected from sites separate from the initial biopsy site. All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Tissue Collection and Histological and Morphometric Analyses
Adult mice were euthanized; their hair shaved and skin from the ear and back was processed for either frozen or paraffin sectioning. For paraffin sectioning, skin was placed in 10% buffered formalin (Surgipath Medical Industries, Richmond, IL), overnight at 4°C before dehydration and embedding (Sakura Finetech, Torrance, CA). For frozen sectioning, skin was either fixed in the non-cross-linking fixative Histochoice (Amresco, Solon, OH) for 4 hours at 4°C, transferred to 5% sucrose for 1 hour at 4°C and placed in 20% sucrose overnight at 4°C or embedded fresh in Tissue Fixation Media in TBS (TFM; Triangle Biomedical), and then flash frozen in liquid nitrogen. Additional pieces of ear and back skin from the same animal were flash frozen in liquid nitrogen and stored at −80°C for use in protein and RNA experiments.
H&E staining was completed on 5-μm thick paraffin sections using standard protocols.10 Immunohistochemistry was performed on TFM-embedded frozen skin sectioned at 8 μm, using antibodies specific for CD4, CD8, CD11c, Gr-1 (BD Biosciences, San Jose, CA), F4/80 (eBioscience, San Diego, CA), Ki-67 (DAKO, Carpinteria, CA), Loricrin (Covance Research Products, Denver, PA), and MECA-32 (MECA-32, Developmental Studies Hybridoma Bank, Iowa City, Iowa), or on 5-μm thick paraffin-embedded sections using high temperature antigen retrieval for VEGF (clone C-1, Santa Cruz Biotechnology, Santa Cruz, CA), Stat3 and phopho-Stat3 (Cell Signaling Technology, Danvers, MA), or matched isotype controls: rat IgG2a, mouse IgG1κ (BD Pharmingen), rat IgG2b, and hamster IgG (Serotec, Raleigh, NC). Antibodies and isotype controls were detected using either rabbit anti-rat IgG biotinylated, goat anti-rabbit IgG biotinylated (Vector Laboratories, Burlingame, CA), or rabbit anti-hamster IgG biotinylated (Southern Biotech, Birmingham, AL), amplified with Avidin/Biotinylated Enzyme Complex (ABC, Vector) and were visualized using the enzyme substrate diaminobenzidine (Vector). Gr-1 was visualized using ABC-Alkaline Phosphatase (ABC-AP, Vector) and Vector Red substrate (Vector). Slides were counterstained with hematoxylin. Images were captured using a Leica DM L82 microscope with an attached Q Imaging MicroPublisher 3.3 Mega Pixel camera and Q-capture Pro software.
Epidermal thickness was quantified on the H&E stained sections using Image-Pro Plus (MediaCybernetics, Bethesda, MD). For each animal, 20 measurements were taken from four different fields of view for each of four individual sections analyzed (320 total measurements/animal). Epidermal thickness was measured from the stratum basale to stratum granulosum, excluding the stratum corneum and hair follicles for each animal.
Blood vessel analyses was completed on MECA-32-stained tissue sections and included quantifying the number of blood vessels, the average blood vessel length, and the average vessel area in the dermis, excluding the hypodermis. Photographs were taken and image analyses completed in a blinded fashion using an automated Metamorph software program (Molecular Devices, Sunnyvale, CA). Background staining was minimal therefore color thresholds were not altered between samples. Post analysis confirmation of blood vessel analysis demonstrated no false positive identification of blood vessels. As before, four fields of view from four individual sections were analyzed per animal with at least 200 individual blood vessels included in the final measurements.
RNA Isolation, Quantitative Real-Time Reverse Transcription-PCR and Microarray
Ear and back skin were homogenized using a Mikro-Dismembrator S (Sartorius Stedim Biotech, Edgewood, NY) and RNA isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. First strand cDNA synthesis was achieved using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Quantitative real-time reverse transcription (RT)-PCR was performed using Taqman technology from Applied Biosystems on an ABI Prism 7700 Sequence Detector. Probes and primers for Tie2, S100A8, S100A9, cathelicidin, β-defensin, interleukin (IL)-1α, IL-6, tumor necrosis factor (TNF)α, and 18S were obtained from Applied Biosystems. Expression levels were calculated relative to 18S using the comparative Ct method (ΔΔCT). RNA was further analyzed using a Th17-specific Autoimmunity and Inflammation PCR Array (SuperArray, Frederick, MD).
Affymetrix Microarray was performed on pooled back skin samples of KC-Tie2 mice (n = 3/group). Groups tested included KC-Tie2 mice with an established phenotype, KC-Tie2 mice 96 hours after doxycycline removal (ie, gene repression occurred throughout gestation into adulthood, the gene was turned on, and 96 hours later the tissue collected), and littermate controls for each group. Equivalent amounts of pooled sample RNA was provided to the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center at Case Western Reserve University where the samples were each processed on a mouse Genome 430 2.0 chip containing 45,101 probe sets. Gene expression and data analyses were performed as described previously11 and with Ingenuity Pathways Analysis (Ingenuity Systems, Inc., Redwood City, CA). Individual genes were validated using quantitative real time RT-PCR.
Protein Analyses via Western Blotting and Enzyme-Linked Immunosorbent Assay
Separate ear and back skin samples taken from the same mice as used for histology, immunohistochemistry, and RNA analyses were homogenized as above. Protein was isolated using PLC++ buffer (50 mmol/L HEPES pH 7.5, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10 mmol/L NaPPi, 100 mmol/L NaF, 1 mmol/L sodium orthovanadate, and 1 miniprotease inhibitor cocktail tablet [Roche Applied Science, Indianapolis, IN]) and quantified as described previously.5,12 For Western blotting analyses, 20 μg of protein/sample was separated on an SDS-polyacrylamide electrophoresis gel and transferred onto polyvinylidene difluoride membrane (Millipore, Temecula, CA). Membranes were blocked in 5% milk in TBS-T (20 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 0.1% Tween-20) and probed with antibodies specific for Tie2 (goat), and VEGF (rabbit) (BD Bioscience and Santa Cruz Biotechnology, Inc., Santa Cruz, CA), washed, incubated with rabbit anti-goat, and goat anti-rabbit IgG horseradish peroxidase secondary antibodies (Zymed Laboratories, Inc., South San Francisco, CA), washed again, and visualized with Immobilon Western HRP substrate (Millipore, Temecula, CA).
Further analyses of the following growth factors and cytokines were completed by enzyme-linked immunosorbent assay (ELISA) using the suggested protocol of the manufacturer: VEGF, IL-4, IL-12p70, IL-17, IL-22, interferon (IFN)γ (R&D Systems, Minneapolis, MN), IL-12p40 (BD Biosciences), and IL-23p19/40 (eBiosciences, San Diego, CA).
Statistical Analysis
All data are represented as mean ± SEM. Between-group comparisons were analyzed using an unpaired, two tailed Student’s t-test and statistical significance was defined as P < 0.05.
Results
EC-Tie2 and KC-Tie2 Mice Have Increased Angiogenesis but Distinct Vascular Phenotypes
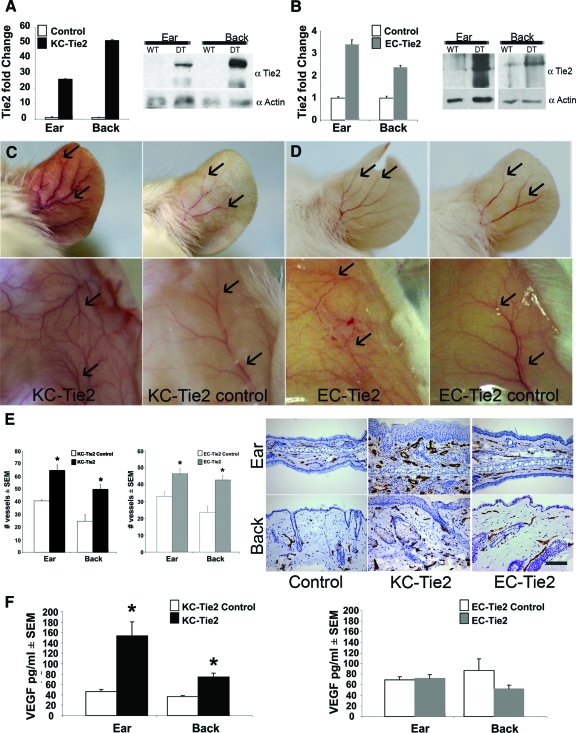
To gain insight into the role of EC- versus KC-derived overexpression of Tie2, we overexpressed Tie2 in either an EC- or KC-specific manner, using a doxycycline-based binary transgenic expression system. EC- and KC-specific Tie2 gene expression was accomplished using the well-characterized Tie1tTA and K5tTA promoter-specific driver lines of mice6,8 which were bred with the TetosTie2 mice that were used previously.5 Increases in Tie2 gene expression were confirmed in the EC-Tie2 and KC-Tie2 double transgenic mice (mice genotypically positive for both tTA and Tetos genes) using real time RT-PCR and Western blotting (Figure 1, A–B). KC-Tie2 and EC-Tie2 mice had a 26- and 3.4-fold increase in Tie2 mRNA in ear skin, respectively; and 52- and 2.4-fold increase in back skin when compared with their littermate controls. This increase was confirmed at the protein level (Figure 1, A–B). At the visual level, increased angiogenesis in the ears of both the KC-Tie2 and EC-Tie2 mice was apparent (Figure 1, C–D). The vascular changes exhibited patterns unique to each mouse line. For example, KC-Tie2 mouse ears were erythematous and contained larger blood vessels that were more highly branched. EC-Tie2 ear skin was not erythematous and the vessels were similar size as control littermates, although an increase in the number of vessel branches was observed. A similar trend was observed for vessels in both dorsal and ventral superficial fascia (Figure 1, C–D). KC-Tie2 mice had larger, more highly branched vessels, whereas EC-Tie2 mice showed only increases in vessel branching (Figure 1D). The vascular patterning was distinct for both lines of mice compared with littermate controls.
Figure 1.
EC-Tie2 and KC-Tie2 mice have distinct phenotypes. Real time RT-PCR and Western blot analysis confirmed increases in Tie2 RNA and protein in ear and back skin of KC-Tie2 (A) and EC-Tie2 (B) double transgenic mice (DT) compared with littermate controls (WT)(n = 6–8 for KC-Tie and littermate controls; n = 4 for EC-Tie2 and littermate controls). KC-Tie2 (C) and EC-Tie2 (D) mice showed increased angiogenesis (arrows) in the ear and superficial fascia compared with control littermates; only KC-Tie2 animals had erythematous ears (C). Endothelial cells of blood vessels in ear and back skin were stained using an antibody specific for MECA-32 (E). KC-Tie2 and EC-Tie2 mice have increased dermal angiogenesis compared with littermate controls (E; n = 4–6 each). KC-Tie2 but not EC-Tie2 mice have increased expression of VEGF protein compared with littermate controls as measured using ELISA (F) (KC-Tie2 n = 10–11; EC-Tie2 n = 7). *P < 0.05 compared with littermate controls. Scale bar = 100 μm.
To quantify the vascular changes in the skin, thin sections collected from ear and back skin were stained using antibodies against the pan EC marker, mouse EC antigen (MECA-32; Figure 1E). KC-Tie2 ears contained 59% more blood vessels than littermate controls (Figure 1E; 64.7 ± 4.2 KC-Tie2 vs. 40.6 ± 0.8 controls, n = 4 each; P = 0.005). KC-Tie2 back skin (Figure 1E) also showed a 102% increase in the number of vessels (49.8 ± 3.8 KC-Tie2 vs. 24.6 ± 4.9 controls, n = 4 each; P = 0.0067). Consistent with the visual increase in the number of vessel branches in ear skin at the gross level in EC-Tie2 animals (Figure 1D), histological analysis of MECA-stained sections confirmed the increase in the number of vessels in the ear (41%, 46.4 ± 2.8 EC-Tie2 vs. 32.8 ± 2.9 controls, n = 4 and 6 respectively; P = 0.013; Figure 1E). EC-Tie2 mice also showed an 82% increase in the number of vessels in back skin (Figure 1E; 42.7 ± 2.8 EC-Tie2 vs. 23.5 ± 3.4 controls, n = 4 and 6 respectively; P = 0.009).
KC-Tie2 but Not EC-Tie2 Mice Have Increased VEGF Protein
The increase in angiogenesis in the dermis and superficial fascia observed in the KC-Tie2 mice where expression of a transmembrane tyrosine kinase receptor had been confined to the epidermis suggested that changes may have occurred in KC-derived secreted growth factor(s) that altered the dermal milieu. Using an ELISA approach we evaluated changes in VEGF protein expression, a likely candidate to stimulate vascular remodeling. KC-Tie2 mice had a ∼3-fold increase in VEGF in ear skin (Figure 1F; 154 ± 27pg/ml KC-Tie2 vs. 46 ± 5pg/ml controls, P = 0.02) and a 1.5-fold increase in VEGF in back skin (74 ± 7pg/ml KC-Tie2 vs. 36 ± 3pg/ml controls, P = 0.003). Immunohistochemical staining using an antibody specific for VEGF confirmed the increase (see supplemental Figure 1 at http://ajp.amjpathol.org). ELISA analyses of EC-Tie2 skin showed no change in VEGF protein expression compared with littermate controls (Figure 1F; and supplemental Figure 1 at http://ajp.amjpathol.org).
KC-Tie2 but Not EC-Tie2 Mice Spontaneously Develop a Phenotype Analogous to Human Psoriasis
EC-Tie2 mice did not develop a psoriasiform phenotype spontaneously, nor did wounding, tape-stripping, or treating both topically and subcutaneously with oxazolone elicit plaque formation, or induce an acanthotic reaction at the histological level, despite the increased influx of immunocytes combined with increased angiogenesis (data not shown).
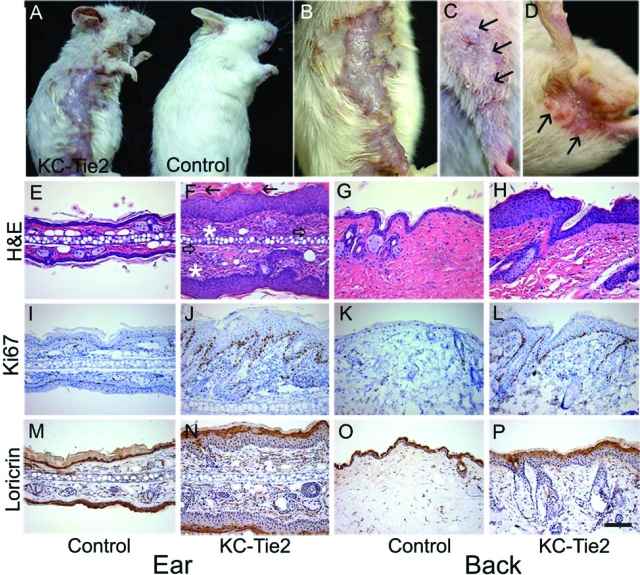
KC-Tie2 mice and littermate controls were indistinguishable from each other at the time of birth; however by the time of weaning (day 21) KC-Tie2 animals developed erythematous ears and increases in the subcutaneous vasculature became apparent (Figure 1C). As early as 8 weeks of age, but as late as 6 months of age, other areas of skin, including the dorsal and ventral surfaces (Figure 2, A–B), bilateral elbows (Figure 2C), and skin surrounding the genital region (Figure 2D), began to develop reddened, scaly, hyperkeratotic lesions. These lesions progressed with time to develop into raised plaques (Figure 2, A–B).
Figure 2.
KC-Tie2 mice spontaneously develop a psoriasis phenotype. KC-Tie2 mice develop raised plaques on their torso (A, B) and bilaterally flakey, scaly skin on their elbows (C) and surrounding their genitals (D). H&E stained sections of ear (E, F) and back (G, H) skin demonstrate acanthosis, loss of the granular cell layer, thickening of the interfollicular epidermal layers, confluent parakeratotic scale (closed arrows), increased dermal angiogenesis (open arrows), and extensive inflammatory infiltrate (asterisks) in KC-Tie2 mice (F, H) compared with control mice (E, G). Increases in Ki-67 staining were evident in ear (I, J) and back (K, L) skin from KC-Tie2 mice (J, L) compared with littermate controls (I, K). Decreases in Loricrin staining in ear (M, N) and back (O, P) skin were found in KC-Tie2 mice (N, P), as compared with control mice (M ,O). Scale bar = 100 μm.
Interestingly, only two observations of KC Tie2 expression in murine or human skin have been reported.5,13 In our hands, we have not found expression of Tie2 in mouse or human cultured KCs or in mRNA isolated from laser capture dissected KCs from involved psoriatic skin (data not shown). Other investigators have also confirmed a lack of Tie2 expression in human and murine KCs (Dr. Michael Detmar; personal communication). Although the initial trigger for psoriasis development has yet to be identified, the skin response and downstream phenotypic characteristics are quite similar between murine models and human psoriasis. Similarly, in this mouse model, Tie2 in KCs appears to initiate a series of events that results in analogous cascades of events, ultimately leading to the development of plaque.
The KC-Tie2 animals showed no signs of hair loss other than in regions containing the most severe plaque. Of 180 KC-Tie2 mice evaluated thus far, 73% (131 mice) exhibited both ear and back phenotypes, while 22% of the animals (40 mice) developed the ear phenotype alone, and 5% developed no phenotype at all (9 mice). Interestingly, animals with no overt phenotype still showed a ∼26-fold increase in Tie2 gene expression compared with control littermates (data not shown). KC-Tie2 mice were fertile and could successfully reproduce. Nails were kept trimmed on all animals at time of weaning and beyond to prevent scratching and excoriation of the ears.
Histological examination of affected ear and back skin showed hyperplasia of the epidermis (acanthosis), a loss of the granular cell layer, thickening of the interfollicular epidermal layers, confluent parakeratotic scale, increased dermal angiogenesis, and extensive inflammatory infiltrate (Figure 2, E–H). Image analyses of the epidermis revealed a 3.2- and 3.1-fold increase in epidermal thickness between KC-Tie2 and control littermates in ear and back, respectively (ear; 67 ± 4.9 KC-Tie2 vs. 16.5 ± 1.8 controls, back; 55 ± 2.9 KC-Tie2 vs. 13.5 ± 1.1 controls, n = 14 controls, n = 16 KC-Tie2, P < 0.001). Additional analyses of the epidermal phenotype showed characteristic increases in Ki-67 (Figure 2, I–L), and loss of loricrin staining (Figure 2, M–P), indicative of increased mitosis and loss of terminal KC differentiation, respectively.
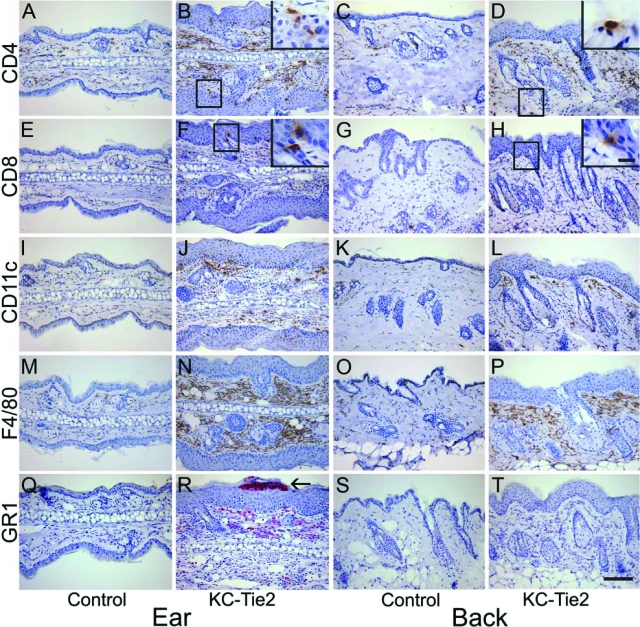
Systematic evaluation of the inflammatory infiltrate in the KC-Tie2 mouse skin revealed increased numbers of CD4+ T cells primarily in the dermis of affected ear and back skin (Figure 3, A–D), CD8+ T cells in the epidermis (Figure 3, E–H), presence of CD11c+ myeloid dendritic cells (Figure 3, I–L), and F4/80+ macrophages (Figure 3, M–P), with many lining the dermal epidermal junction, representative of lining macrophages.14 In addition, GR1+ granulocytes were observed in the dermis and micro abscesses in the epidermis of ears (Figure 3, Q–T), most likely representative of neutrophils, as no F4/80 staining was observed in the epidermis. Increased numbers of mast cells and no difference in the number of eosinophils were found between KC-Tie2 and control littermates (data not shown). The presence of these immunocytes represents the classic immunophenotypic profile of human psoriatic lesions.
Figure 3.
Inflammatory cell infiltrate in KC-Tie2 mice contains T cells, dendritic cells, macrophages and neutrophils. Skin from control ear (A, E, I, M, Q) and back skin (C, G, K, O, S), and KC-Tie2 ear (B, F, J, N, R) and back skin (D, H, L, P, T) was stained using immunohistochemical methods with antibodies against CD4+ T cells (A–D), CD8+ T cells (E–H), CD11c+ dendritic cells (I–L), F4/80+ macrophages (M–P), and Gr-1+ granulocytes (Q–T). Higher magnification insets correspond to boxed areas. Scale bar = 100 μm, 10 μm in insets. Arrow in R indicates a micro abscess.
Molecular and Biochemical Characteristics of the KC-Tie2 Psoriatic Phenotype Mimics Human Disease
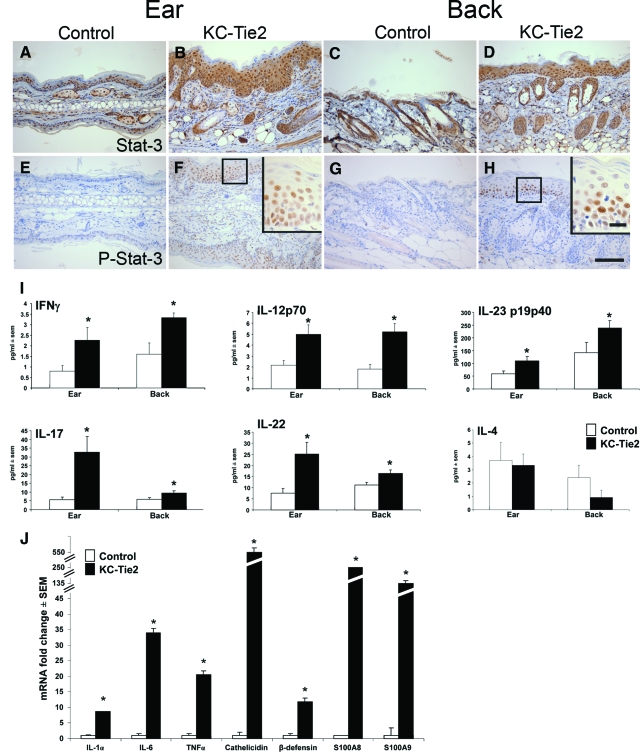
A large number of cytokines, chemokines, and transcription factors have been proposed to contribute to the pathogenesis of psoriasis,15,16 many of which have been used in attempts to engineer a mouse model representative of this complex, multifaceted human disease.1,17,18,19 We used immunohistochemistry, ELISA, microarray, and real-time RT-PCR approaches to quantify changes in our mouse model of several of the cytokines, transcription factors and host defense genes known to be up-regulated and involved in human psoriasis. We observed significant increases in Stat3 protein in KC-Tie2 mice compared with littermate controls (Figure 4, A–D) but more importantly, were able to show a considerable up-regulation of naturally occurring Stat3 phosphorylation in the epidermis of KC-Tie2 animals compared with littermate controls (Figure 4, E–H). Stat3 activity has previously been shown to be critical in human disease, and engineered over expression of active Stat3 has been used to create one of the more relevant mouse models of human psoriasis.17 In addition to phosphorylation of Stat3, significant increases in Th1-related cytokines including IFNγ, IL-12, and IL-23 were found in the KC-Tie2 mice when compared with littermate controls while the Th2-related cytokine IL-4 remained unchanged (Figure 4I). These profiles are consistent with the cytokine and T cell profile observed in human psoriasis. Moreover, Th17-derived cytokines, specifically IL-17 and IL-22 were also up-regulated (Figure 4I).
Figure 4.
KC-Tie2 mouse skin has increased Stat3 activity and a cytokine profile consistent with human psoriasis. Stat3 (A–D) and phospho-Stat3 (E–H) are up-regulated in KC-Tie2 ear (B, F) and back skin (D, H), as compared with control ear (A, E) and back skin (C, G). ELISA analyses of a panel of cytokines shows significant increases in Th1-related cytokines (I), including IFNγ, IL-23p19p40, and IL-12p70. Th17-derived cytokines, IL-17 and IL-22, were also up-regulated in ear and back skin of KC-Tie2 mice as compared with controls. The Th2-related cytokine, IL-4 remained unchanged in both ear and back skin (I) (n = 6 to 8). Real time RT-PCR results show significant upregulation of IL-1α, IL-6, TNFα, cathelicidin, β-defensin, S100A8, and S100A9 in KC-Tie2 back skin compared with littermate controls (J)(n = 3–4). Higher magnification insets correspond to boxed areas. *P < 0.05 compared with littermate controls. Scale bar = 100 μm, 10 μm in insets.
To further characterize the molecular phenotype, gene expression profiling of the skin from KC-Tie2 mice was performed using an Affymetrix Mouse Genome Array. Analyses of genes that were either increased or decreased at least twofold were included. Independent confirmation of increased Tie2 expression in KC-Tie2 mice was reported in the array, such that Tie2 was found to be increased by 64-fold (P = 0.0003). Other changed genes included increased expression of three critical pro-inflammatory cytokines found in human psoriasis: TNFα (3-fold increase, P = 0.002), IL-6 (4-fold increase, P = 0.00002), and IL-1α (5-fold increase, P = 0.00004), which were validated with real time RT-PCR (see below). Additional markers that were found to be differentially regulated in our murine model that are also altered in human psoriasis included: increases in Gro1 (CXCL1; 10-fold increase, P = 0.00002), CSF2 (11.8-fold increase, P = 0.00002), and K16 (6.5-fold increase, 0.00002), and decreases in caveolin (2-fold decrease, P = 0.00002) and leptin (4-fold decrease, 0.00004). Other molecules recently reported to be involved in the development and maintenance of human psoriasis, included increases in the chemotactic proteins, S100A8 and S100A9 (13- and 29.9-fold increase, respectively, P = 0.00002) and increases in host defense genes, such as cathelicidin (40-fold increase, P = 0.0002), mouse β-defensin 3 (Mbd3, 12-fold increase, P = 0.0002) and secretory leukocyte protease inhibitor (Slpi; 12.1-fold increase, P = 0.00002). RT-PCR validation of IL-1α, IL-6, TNFα, cathelicidin, Mbd3, S100A8, and S100A9 confirmed a 9-, 34-, 21-, 549-, 12-, 254-, and 132-fold increase compared with littermate controls, respectively (Figure 4J).
To examine the molecular events driving the development of the phenotype, we repressed gene expression in a separate litter of mice from the point of conception until adulthood by including doxycycline in the drinking water and then removing it, thereby reintroducing gene expression. Within 3 days, S100A8 (2.3-fold increase, P = 0.001), CXCL7 (4.6-fold increase, P = 0.0002), and epiregulin (2-fold increase, P = 0.00002) were significantly up-regulated whereas leptin was found to already be down-regulated (2.3-fold decrease, P = 0.0005).
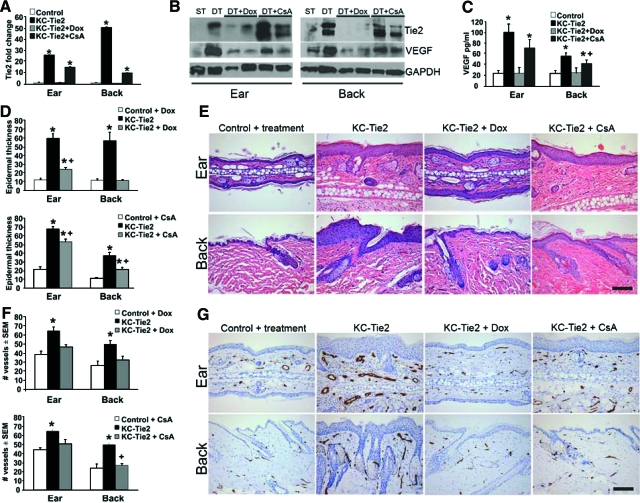
KC-Tie2 Ear and Back Skin Respond Differently to Gene Repression and Systemic CsA Treatment
To determine whether the phenotype was permanent once it was established or if it was reversible, mice were treated for a 4-week period with either doxycycline (n = 4), to repress Tie2-transgene expression, or with the commonly used psoriasis systemic immunosuppressant, cyclosporin A (CsA; 80 mg/kg of CsA gavage, three times weekly, n = 4). The original back skin phenotype, including the flaky skin and overt plaques were reversed following either treatment course, and the dorsal and ventral surfaces of the skin were indistinguishable from littermate controls. The ear skin phenotype improved however low levels of erythema continued to be present in both treatment paradigms, with the CsA treatment appearing to be less effective than the doxycycline (data not shown). Real time RT-PCR (Figure 5A) confirmed the decreases in Tie2 expression in ear and back skin following exposure to doxycycline. Treatment with CsA also resulted in decreased Tie2 mRNA expression, although these levels remained 15-fold higher than littermate control animals. Western blotting in two representative animals demonstrated that doxycycline-mediated gene repression decreased levels of Tie2 protein (KC-Tie2 + Dox). KC-Tie2 mice treated with CsA (KC-Tie2 + CsA) expressed Tie2 protein levels that equaled or exceeded the expression of Tie2 found in the representative untreated KC-Tie2 mouse ear and back skin. Analysis of VEGF expression following both treatment paradigms showed a complete return to basal levels in KC-Tie2 mice treated with doxycycline in both back and ear skin, whereas mice treated with CsA showed significant reductions in VEGF protein, but these levels were still significantly elevated compared with littermate controls (Figure 5, B–C).
Figure 5.
Doxycycline and CsA treatments lead to reversal and improvement in the psoriatic phenotype, respectively. Real time RT-PCR (A) and Western blotting (B) analyses of Tie2 expression following doxycycline (Dox) or cyclosporin A (CsA) treatment (n = 4 each). Tie2 expression returns to control levels following Dox-mediated gene repression at both RNA and protein levels, whereas CsA treatment leads to decreases in Tie2 RNA in both ear and back skin, but no change in Tie2 protein in back skin only. Western blot (B) displays representative samples from littermate control (ST, n = 1), KC-Tie2 (DT, n = 1), KC-Tie2 + Dox (DT+Dox, n = 2), KC-Tie2 + CsA (DT+CsA, n = 2). Western blot (B) and ELISA (C) analyses of VEGF protein show return to baseline levels in ear and back skin of Dox treated KC-Tie2 mice. CsA treatment lead to reductions in VEGF compared with untreated animals, however these levels remained significantly higher than control mice. Representative H&E stained ear and back skin (E) and epidermal thickness quantification of control mice post treatment, KC-Tie2 mice before treatment and KC-Tie2 mice following 4 weeks of Dox exposure or CsA administration (D). Quantification of MECA-32-stained endothelial cells from ear and back skin (G) with corresponding analysis of blood vessel number for control mice post treatment, KC-Tie2 mice pretreatment and KC-Tie2 mice following 4 weeks of Dox or CsA exposure (F). *P < 0.05 compared with littermate controls. +P < 0.01 compared with KC-Tie2 mice before treatment. Scale bar = 100 μm.
To determine the histological effects of our treatment approaches, a longitudinal comparison of acanthosis was completed by evaluating epidermal thickness in ear and back skin taken from the same mice before and after treatment. Doxycycline treatment resulted in a nearly complete reversal of acanthosis in back skin of KC-Tie2 mice and a significant reduction in epidermal thickness and edema in the ear. Mice treated with CsA showed significantly reduced epidermal thickness in back and ear skin, however the epidermis remained significantly thicker than control littermates (Figure 5, D and E). Interestingly, two of the four animals treated with CsA showed 100% resolution of acanthosis in the back skin, whereas the remaining half only showed improvement, explaining the variability in the overall observations. This trend was evident only in back skin. An additional observation was the sustained edema present in the CsA-treated ear skin, which failed to resolve in any of the treated mice (Figure 5, E and G), perhaps reflecting the continued elevation in VEGF (Figure 5, B and C). The difference we observe in resolution of the skin phenotype between ear and back skin may reflect differences in the tissue anatomy, such that ear skin contains a layer of cartilage instead of the hypodermis and muscle layer found in mouse back skin and these differences may result in lymphocytic compartmentalization making ear tissue more resilient to treatment.
Analyses of the angiogenic phenotype following both doxycycline and CsA treatment revealed a return to control levels for the number of MECA+ stained blood vessels in ear and back skin (Figure 5, F–G).
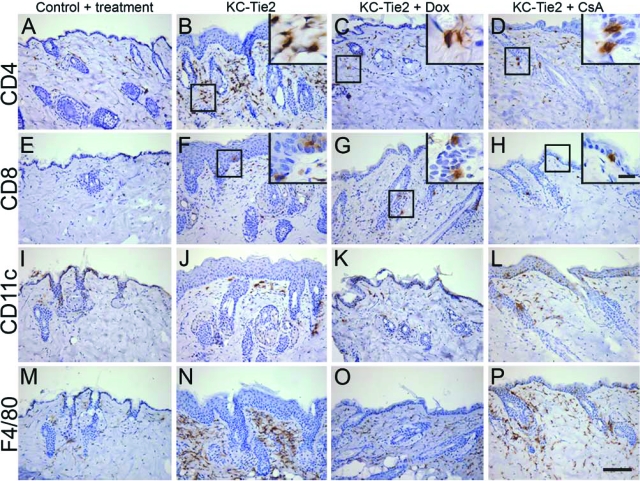
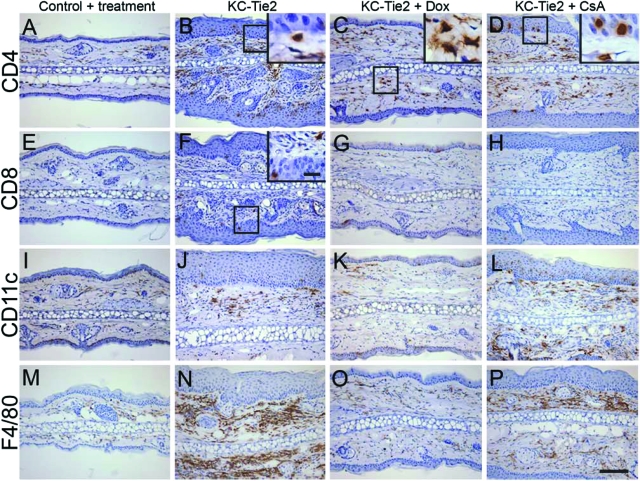
Our initial characterization of the inflammatory infiltrate and cytokine profiles revealed a striking similarity to that observed in human psoriasis, including increases in CD4+, CD8+ T cells, dendritic cells, and macrophages along with significantly elevated Th1- and Th17-derived cytokines (Figure 3). Following 4 weeks of doxycycline exposure, T cell, dendritic cell, and macrophage numbers were reduced in KC-Tie2 back skin (Figure 6, A–C, E–G, I–K, and M–O). For mice treated with systemic CsA, a classic T cell mediated target, T cells were also found to be decreased, although there still appeared to be more T cells present when compared with littermate controls (Figure 6, D and H). The number of dendritic cells present remained high, and only a small decrease in macrophages was seen in KC-Tie2 back skin consistent with the idea that the maintenance of acanthosis may have a T cell independent component (Figure 6, L and P). In ear skin, where the phenotype failed to improve to the same degree as the back skin for both doxycycline and CsA treated animals, numerous CD4+ T cells were still evident in the dermis and epidermis under both treatment conditions (Figure 7, A–D), although CD8+ T cells appeared to decrease to control levels (Figure 7, E–H). Interestingly, for KC-Tie2 ears that showed significantly reduced acanthosis (doxycycline treated), dendritic cell and macrophage numbers returned to control levels (Figure 7, I–K, M–O), whereas in CsA treated mice, where the ear acanthosis improved only minimally, dendritic cell and macrophage numbers appear to stay elevated (Figure 7, L and P).
Figure 6.
Immunocyte profile changes in back skin of KC-Tie2 mice treated with doxyclycline or CsA. CD4+ T cell (A–D), CD8+ T cell (E–H), Cd11c+ dendritic cell (I–L), and F4/80+ macrophage (M–P) staining in back skin of control animals (A, E, I, M), KC-Tie2 mice before treatment (B, F, J, N), and KC-Tie2 mice treated with doxycycline (C, G, K, O) or CsA (D, H, L, P). Higher magnification insets correspond to boxed areas. Scale bar = 100 μm, 10 μm in insets.
Figure 7.
Immunocyte profile changes in ear skin of KC-Tie2 mice treated with doxycycline or CsA. CD4+ T cell (A–D), CD8+ T cell (E–H), Cd11c+ dendritic cell (I–L), and F4/80+ macrophage (M–P) staining in ear skin of control animals (A, E, I, M), KC-Tie2 mice before treatment (B, F, J, N), and KC-Tie2 mice treated with doxycycline (C, G, K, O) or CsA (D, H, L, P). Higher magnification insets correspond to boxed areas. Scale bar = 100 μm, 10 μm in insets.
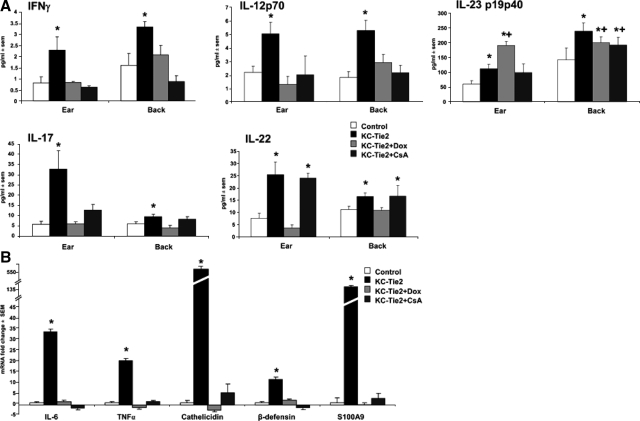
Following treatment for 4 weeks with either doxycycline or CsA, protein levels of IFNγ, IL-17, and the p70 heterodimer of IL-12 were all reduced to control levels in both ear and back skin (Figure 8A). IL-22 expression levels in ear and back skin also returned to basal levels in doxycycline treated mice, where acanthosis improved and antigen presenting cell (APC) numbers were reduced to control levels; in contrast IL-22 expression remained elevated in CsA-treated animals, perhaps reflective of the continued presence of macrophages, dendritic cells, and/or CD4+ T cells. Similar sustained cytokines levels were found for the heterodimer form of IL-23, with the overall trend reflecting sustained expression of IL23p19/p40 across all groups even following treatment (Figure 8A). Of further interest was the complete return to baseline levels for IL-6 and TNFα, and for the host defense genes cathelicidin, β-defensin and S100A9 mRNA following either CsA or doxycycline treatment (Figure 8B).
Figure 8.
Cytokine regulation following treatment with doxycycline or CsA. IFNγ, IL-17, and IL-12p70 protein expression in ear and back skin measured using ELISA returns to control levels following doxycycline (n = 4 each) or CsA treatment (n = 4 each) (A). IL-23p19p40 levels significantly decreased in back skin following doxycycline and CsA treatment but remained elevated as compared with littermate controls. In contrast, IL-23p19p40 expression in ear skin increased from that of KC-Tie2 levels following doxycycline treatment. IL-22 expression in ear and back skin decreases to baseline levels following gene repression using doxycycline but not CsA treatment (A). Real-time RT-PCR analysis of IL-6, TNFα, cathelicidin, β-defensin, and S100A9 expression in back skin harvested from KC-Tie2 mice treated with doxycycline or CsA for 4 weeks showed a complete return to baseline expression levels (B). *P < 0.05 compared with littermate controls, +P < 0.05 compared with KC-Tie2 pretreatment.
Discussion
We initially sought to determine whether either EC- or KC-specific overexpression could be solely responsible for the development of the psoriasiform phenotype previously reported in the KC-EC–Tie2 mouse5,7 and demonstrate here that KC, and not EC Tie2 overexpression is responsible for the development of the psoriasiform phenotype. Despite an increase in subcutaneous and dermal vascularization, and even in the presence of a robust inflammatory response, EC-Tie2 overexpressing mice did not develop acanthosis, suggesting that regardless of the state of vascularization, the presence of more blood vessels is not, on its own, or in combination with sustained inflammation, sufficient to cause the development of a psoriasiform phenotype. This contradicts findings by two other groups who demonstrated VEGF overexpression in KCs, results in increased dermal vascularization, followed by the development of a VEGF-dependent psoriasis-like phenotype. In one case,3 the phenotype developed after wounding young, phenotypically normal mice (Koebner phenomenon) or spontaneously 6 months into adulthood; in the other,4 psoriasis-like characteristics developed only following induction of a delayed type hypersensitivity reaction elicited by treatment with oxazolone. In both cases, the increase in vascularization preceded acanthosis; and introduction of an acute inflammatory reaction was sufficient to drive the development of the epidermal hyperplasia. Despite the increase in vascularization we observed in the EC-Tie2 animals, we were not able to elicit psoriasiform changes. It is possible that the observed increase in angiogenesis was insufficient, or that VEGF protein did not significantly increase in these mice, thereby reducing the amounts of vascular leakiness, although we believe this to be unlikely, as we have also engineered a K5tTA-Tetos VEGF mouse line, and these mice despite increased VEGF, more dermal angiogenesis, and increased vessel leakiness also fail to develop a psoriasiform phenotype (data not shown). The lack of significant increases in angiogenesis (ie, increased vessel number and area) is also unlikely to explain why EC-Tie2 mice failed to develop psoriasiform characteristics, as similar numbers of blood vessels were found in back skin of EC-Tie2 as in KC-Tie2 mice; although the vessels themselves were smaller. Whether the addition of VEGF to our EC-Tie2 mice, in addition to inflammatory stimulus could drive the development of the psoriasiform phenotype was not tested.
The overexpression of Tie2 in KCs leads to the robust spontaneous development of a psoriasis-like phenotype; with distinct differences from the previously published KC-EC–Tie2 mouse (Table 1 and supplementary Table 1 at http://ajp.amjpathol.org), with the prior KC-EC-Tie2 representing a more Th2 skewed, atopic dermatitis like phenotype; while our KC-Tie2 mouse is Th1 and Th17 skewed, and is more representative of psoriasis.5,7 Our KC-Tie2 murine model displays numerous hallmarks of human disease and provides another example where disturbance of the intrinsic KC balance causes psoriasis-like skin inflammation (for review1,4,17). Our examination of the KC-Tie2 mouse suggests that multiple starting points for psoriasiform skin changes may exist and that once the cycle is initiated or triggered; the physical, cellular and immunological changes are the same. Therefore, it is likely that the same cells and molecules that confer and sustain human psoriasis are up-regulated in our animal model. Further, examination of the molecular signaling pathways altered in our model may elucidate key signaling pathway changes associated with the initiation and/or maintenance of the psoriatic phenotype.
Table 1.
Comparison between the KC-Tie2 and KC-EC-Tie2 Mouse Models
| Characteristic | KC-Tie2 mouse | KC-EC-Tie2 mouse |
|---|---|---|
| Physical characteristics | ||
| Demarcated plaques acquired after birth | Yes | Yes (whole body, no demarcation, develops neonatally) |
| Koebner | Yes (in C57Bl/6 background) | Yes |
| Involvement of nails/toes | Yes | Yes |
| Auspitz sign | Yes | Not reported |
| Normal lifespan, healthy weight | yes | No (failure to thrive) |
| Epidermal differentiation | ||
| Confluent hyperkeratosis plus parakeratosis | Yes | Yes |
| Epidermal thickening | Yes | Yes |
| - Aberrant keratin expression | Yes | Not reported |
| - Loss of loricrin expression | Yes | Not reported |
| - Increased Ki67 | Yes | Not reported |
| Intra epidermal micro abscesses | Yes | Yes |
| Rete ridges | Suggestion | Suggestion |
| Presence of PMNs in epidermis | Yes | Yes |
| Vascular proliferation and dilatation | Yes | Yes |
| Increased VEGF | Yes (ELISA) | Yes (ELISA) |
| Increased EGF | Yes (microarray) | Not reported |
| Increased FGF | Yes (microarray) | Not reported |
| Lymphatic proliferation and dilatation | Yes | Not reported |
| Immunophenotype | ||
| T cells in dermis and epidermis | Yes CD4 and CD8 | Yes CD3 |
| Increased presence of macrophages | Yes | Not reported in skin, none present in spleen |
| Increased presence of dendritic cells | Yes | Not reported in skin, none present in spleen |
| Increased presence of eosinophils | No | Increased in skin |
| Th1 (IFNγ, IL12, TNFα)* | Yes | Yes |
| Th2 (IL4, IL9, RANTES, TARC, eotaxin)* | No | Yes |
| Th17* | Yes | No |
| Innate host defense molecules | ||
| β-defensins | Yes | Not reported |
| S100A8/A9 | Yes | Not reported |
| Cathelicidin | Yes | Not reported |
| Response to therapeutics | ||
| CsA responsive | Yes | Yes |
Differences between models are marked in bold. * For additional details on specific cytokine and chemokine breakdown, method of measurement and differences between mouse models, see Supplemental Table 1 at http://ajp.amjpathol.org.
Whether Tie2 expression in KCs is directly responsible for the observed skin phenotype via downstream signaling or indirectly responsible as a result of increasing other growth factors and growth factor signaling is not currently known. Although Tie2 receptor activation is not known to induce mitosis directly, previous studies have shown that Tie2 activation is associated with the generation of reactive oxygen species,20 which can lead to VEGF production,21,22,23 angiogenesis,20 and interactions with cytokines and host defense molecules,24,25 potentially mediating KC proliferation. Alternatively, KC-overexpression of Tie2 may lead to increases in other KC receptor tyrosine kinase signaling pathways, such as epidermal growth factor ligand-receptor interactions and increased MAPK signaling, which would also result in KC proliferation and epidermal acanthosis.26,27,28,29 Identification of these signaling events in this model may provide a preclinical opportunity to test the efficacy of inhibitors of oxidative signaling pathways, such as carbazole or piperazine/piperidine derivatives30,31 or epidermal growth factor32,33,34,35 on psoriatic plaque resolution.
Of particular interest in our model is the development of scale and plaque in regions seen in human patients, including bilateral elbows and surrounding the genital region. At the skin histological level, dermal CD4+ and epidermal CD8+ T cells, dendritic cells, and macrophages were found at increased density and derived cytokines from these cells were significantly elevated. Classic psoriasis related cytokines elevated in our murine model included TNFα, IL-6, IL-1α, IFNγ, and IL-12, in addition to newly described cytokines important in psoriasis pathogenesis such as IL-23, IL-22, and IL-17.15,16,36 As observed for human disease, IL-4, a Th2-related cytokine did not differ between control mice and KC-Tie2 mice.
S100A8/A9 (calgranulin A/B; MRP8/14) are calcium binding proteins whose serum levels have been reported to be elevated in psoriasis. In our model, we detected these potent stimulators of leukocyte trafficking into psoriatic tissue37,38 as early as three days following gene expression. Therefore, KC-derived S100A8/A9 may stimulate immune cell invasion,38 which may then initiate and/or exacerbate dermal inflammation and cytokine production. Recent evidence correlates S100A8/A9 mRNA and IL-22 mRNA levels in psoriasis patients.39 Moreover, S100A8/A9 may serve as the etiological link for the association between psoriasis and cardiovascular disease,40,41,42,43,44 as during initiation and maturation of atheromatous plaques, S100A8/A9 protein expression is increased in microvascular murine ECs45 and macrophages,46 potentially regulating both monocyte transmigration and endothelial activation.47
Increases in cathelicidin (LL37) and β-defensin 3 have been described in human psoriasis, and were found to be up-regulated in our mouse model, perhaps playing a role in attracting and retaining dendritic cells. A recent report by Lande et al,48 suggests that LL37 may serve as the principle trigger of pathogenic IFNα response in psoriatic skin, providing a critical link between microbial defense systems and psoriasis pathogenesis. Their model suggests that skin injury coupled with de-regulated LL37 is capable of eliciting and sustaining DC activation in psoriatic skin, recruiting myeloid cells, and initiating local T cell mediated inflammation and lesion development. Further support for a role for defensins in psoriasis has also recently been reported by Hollax et al, indicating that the higher genomic copies of β-defensin genes, the greater increased risk for psoriasis.49
In our study, repression of Tie2 (doxycycline treatment), reversed acanthosis in the back skin, decreased immunocytes to baseline levels, and lowered expression of inflammatory cytokines. In ear skin, significant reductions in acanthosis were observed, although ears ultimately remained thicker than littermate controls. APCs, including dendritic cells and macrophages were dramatically reduced and cytokines associated with APCs also returned to control levels.
CsA-treated mice showed improved, but not resolved, phenotypes in both back and ear skin. In both back and ear skin, fewer T cells were found but APCs remained high, thus suggesting APCs may play key roles in the development and maintenance of the disease as suggested by several recent reports.18,19,50,51 Despite the presence of remaining APCs following treatment, the cytokine protein levels improved significantly, including a return to control levels for IFNγ, IL-17, and IL-12p70. IL-22 remained elevated, most likely reflecting the ongoing presence of APCs and Th1 cells and could explain the sustained acanthosis in the ear and back, as IL-22 receptors are found on KCs, but not circulating immune cells and can, on its own, induce KC hyperplasia.52 Whether IL-22 inhibition would resolve the phenotype in our animals is unknown, but is possible, as others have recently shown its efficacy in resolving murine psoriasiform dermatitis.53 For both doxycycline- and CsA-treated mice, regulation of IL-23p19p40 was more complex. Doxycycline-treated mice maintained elevated expression of IL-23p19p40 in back and ear skin despite resolution and improvement in IL12/23p40, perhaps indicating decreased p40 homodimer formation (data not shown). Resolution of the phenotype in these mice may reflect the efficacy of therapeutic targeting of p40 expression using anti-p40-antibodies, shown to successfully improve clinical outcomes.54 Improvement of the psoriasis phenotype with decreased IL-12/23 would also be consistent with previous work showing KC-specific overexpression of p40 leads to the development of inflamed skin.55 In CsA treated mice, IL23p19p40 remained increased in back skin (where the phenotype was found to improve), whereas in the ear skin, where minimal improvement in acanthosis was found, IL23p19p40 returned to control levels. Innate immune response markers across both treatment conditions all returned to control levels or lower in back skin (Figure 8B), indicating a correlative, but not necessarily a causative relationship with resolution of the psoriasis phenotype.
In our hands, KC-derived Tie2 cannot be detected under normal or pathological conditions in humans and mice including laser capture microdissection of the basal layers of KCs isolated from involved human psoriasis plaque, uninvolved skin, and control patient skin, combined with real time RT-PCR. Despite these findings, alterations to KC-Tie2 clearly induce signaling events mimicked in human psoriasis including Stat3 phosphorylation and downstream regulation of inflammatory cytokines.
The initial trigger used to generate the phenotype (Tie2 in KCs) may not represent a biological event in human psoriasis; however the downstream events are found in human psoriasis progression and play key roles in the current model. Using Ingenuity® Pathways Analyses, we identified IL-6, NFκB, Fos, Jun (AP1), and Stat signaling as key components of the phenotype in our mouse model. Additional important molecules upregulated before development of an overt phenotype included NFκB, TNFα, TGFβ, and Stat3. Analyses specifically queried for Tie2 related pathways connected Tie2 to S100A8/A9, T cell receptor, Stat3, VEGF, and IL-6 (Tie2-Akt-PKC-S100A8/A9; Tie2-Akt-PKC-TCR-MAPK-Stat; Tie2-Akt-PI3K-Hif1α–Stat3; Tie2-Akt-PI3K-Hif1α-VEGF–IL-6), which in turn lead to identification of downstream components, including cytokine regulation, angiogenesis, and sustained inflammatory reactions. Stat3, VEGF, and IL-6 have all been targeted previously in murine models of psoriasis, and are all up-regulated in human disease.1
Nonetheless, factors favoring our model as a strong mimic of human psoriasis include: spontaneous phenotype development, chronic but reversible, and nonscarring. This model also reflects classic psoriatic immunocyte profiles and their derived cytokines and is responsive to a common psoriasis therapy. The ability to regulate gene expression will allow for temporal gene identification and validation during not only disease progression, but also reversal of the disease and will be highly valuable in identifying the initial trigger(s) of disease progression. This model meets the clinical, histological, immunophenotypic, biochemical, and pharmacological criteria required for an animal model of human psoriasis and therefore will become important for studying pathological mechanisms of psoriasis and pre-clinical testing of new therapeutics.
Note Added in Proof
At the time we submitted and revised this manuscript, we reported that our KC-Tie2 mouse model developed a psoriasisform skin disease phenotype in the absence of any signs of oncogenesis, including animals that were almost 2 years old. We have recently begun to observe spontaneous development of tumors in offspring from a subset of our KC-Tie2 breeding colony, and despite having animals in the colony that are older than 1 year of age with no signs of oncogenesis, we can no longer assert a complete lack of oncogenesis in certain progeny of this animal model. We are currently working at characterizing the genetics underlying these observations.
Supplementary Material
Acknowledgments
The K5-tTA mouse was generously provided by Dr. Adam Glick (Pennsylvania State University) and the TetosTek and Tie1-tTA mice by Dr. Daniel Dumont (Sunnybrook & Women’s Research Center, Toronto, Canada). We thank the SDRC Morphology Core for their technical assistance and Dr. Scott Howell for assistance with image analyses and laser capture microdissection.
Footnotes
Address reprint requests to Dr. Nicole L. Ward, Case Western Reserve University, Departments of Dermatology and Neuroscience, BRB519, 10900 Euclid Ave, Cleveland, OH 44106. E-mail: nicole.ward@case.edu.
Supported by the American Skin Association, a pilot and feasibility grant from the Case Western Reserve University Skin Diseases Research Center (NIH-NIAMS; P30-AR-39750), a career development award from the Dermatology Foundation (NLW), the Murdough Family Center for Psoriasis, and the Psoriasis Center of Research Translation (NIH-NIAMS; 1P50AR055508-01). J.A.W. is supported by training grant T32 GM-08056-23 (NIH-NIGMS). Microarray analyses were completed with support from the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (NIH-NCI; P30-CA43703).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- Conrad C, Nestle FO. Animal models of psoriasis and psoriatic arthritis: an update. Curr Rheumatol Rep. 2006;8:342–347. doi: 10.1007/s11926-006-0063-x. [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Voskas D, Jones N, Van Slyke P, Sturk C, Chang W, Haninec A, Babichev YO, Tran J, Master Z, Chen S, Ward N, Cruz M, Jones J, Kerbel RS, Jothy S, Dagnino L, Arbiser J, Klement G, Dumont DJ. A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice. Am J Pathol. 2005;166:843–855. doi: 10.1016/S0002-9440(10)62305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarao R, Dumont DJ. Conditional transgene expression in endothelial cells. Transgenic Res. 1998;7:421–427. doi: 10.1023/a:1008837410485. [DOI] [PubMed] [Google Scholar]
- Voskas D, Babichev Y, Ling LS, Alami J, Shaked Y, Kerbel RS, Ciruna B, Dumont DJ. An eosinophil immune response characterizes the inflammatory skin disease observed in Tie-2 transgenic mice. J Leukoc Biol. 2008;84:59–67. doi: 10.1189/jlb.0607347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–445. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DC, Hrapchak BB. Detroit,: Battelle Press; Theory and practice of Histotechnology. 1980:p 496. [Google Scholar]
- Zhou L, Askew D, Wu C, Gilliam AC. Cutaneous gene expression by DNA microarray in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2007;127:281–292. doi: 10.1038/sj.jid.5700517. [DOI] [PubMed] [Google Scholar]
- Ward NL, Slyke PV, Dumont DJ. Functional inhibition of secreted angiopoietin: a novel role for angiopoietin 1 in coronary vessel patterning. Biochem Biophys Res Comm. 2004;323:937–946. doi: 10.1016/j.bbrc.2004.08.185. [DOI] [PubMed] [Google Scholar]
- Markham T, Mullan R, Golden-Mason L, Rogers S, Bresnihan B, FitzGerald O, Fearon U, Veale DJ. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy. J Am Acad Dermatol. 2006;54:1003–1012. doi: 10.1016/j.jaad.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Wortmann S, Kaufmann R, Mielke V, Sterry W. A subset of macrophages located along the basement membrane (“lining cells”) is a characteristic histopathological feature of psoriasis. Am J Dermatopathol. 1995;17:139–144. doi: 10.1097/00000372-199504000-00005. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Xin H, Nestle FO, Qin JZ. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568–573. doi: 10.1016/j.clindermatol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–244. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- Stratis A, Pasparakis M, Rupec RA, Markur D, Hartmann K, Scharffetter-Kochanek K, Peters T, van Rooijen N, Krieg T, Haase I. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–2104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, Valo M, Sturk C, Carrillo CO, Sohn A, Cerimele F, Dumont D, Losken A, Williams J, Brown LF, Tan X, Ioffe E, Yancopoulos GD, Arbiser JL. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126:2316–2322. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA: 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999;13:2002–2014. [PubMed] [Google Scholar]
- Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277:33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd SL, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 2007;127:2001–2011. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- Varani J, Lateef H, Fay K, Elder JT. Antagonism of epidermal growth factor receptor tyrosine kinase ameliorates the psoriatic phenotype in organ-cultured skin. Skin Pharmacol Physiol. 2005;18:123–131. doi: 10.1159/000084909. [DOI] [PubMed] [Google Scholar]
- Albanesi C, De Pita O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–588. doi: 10.1016/j.clindermatol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Forsberg S, Ostman A, Rollman O. Regeneration of human epidermis on acellular dermis is impeded by small-molecule inhibitors of EGF receptor tyrosine kinase. Arch Dermatol Res. 2008;300:505–516. doi: 10.1007/s00403-008-0853-2. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kanno H, Watabe D, Akasaka T, Sawai T. The role of heparin-binding EGF-like growth factor and amphiregulin in the epidermal proliferation of psoriasis in cooperation with TNFalpha. Arch Dermatol Res. 2008;300:37–45. doi: 10.1007/s00403-007-0809-y. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Govindarajan B, Battle TE, Lynch R, Frank DA, Ushio-Fukai M, Perry BN, Stern DF, Bowden GT, Liu A, Klein E, Kolodziejski PJ, Eissa NT, Hossain CF, Nagle DG. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Invest Dermatol. 2006;126:1396–1402. doi: 10.1038/sj.jid.5700276. [DOI] [PubMed] [Google Scholar]
- Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen. 2006;9:425–442. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- Neyns B, Meert V, Vandenbroucke F. Cetuximab treatment in a patient with metastatic colorectal cancer and psoriasis. Curr Oncol. 2008;15:196–197. doi: 10.3747/co.v15i4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivin F, Boucher E, Raoul JL. Complete sustained regression of extensive psoriasis with cetuximab combination chemotherapy. Acta Oncol. 2004;43:592–593. doi: 10.1080/02841860410020211. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- Eriksen JG, Overgaard M. Late onset of skin toxicity induced by EGFr-inhibitors. Radiother and Oncol. 2009;90:280–291. doi: 10.1016/j.radonc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60:569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Eue I, Sorg C. The regulatory role of MRP8 (S100A8) and MRP14 (S100A9) in the transendothelial migration of human leukocytes. Pathobiology. 1999;67:230–232. doi: 10.1159/000028098. [DOI] [PubMed] [Google Scholar]
- Liu H, Huang K, Wu Y, Lin N, Li J, Tu Y. The expression of interleukin-22 and S100A7. A8, A9 mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technolog Med Sci. 2007;27:605–607. doi: 10.1007/s11596-007-0533-z. [DOI] [PubMed] [Google Scholar]
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Granath F, Hamsten A, Stahle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol. 2006;54:614–621. doi: 10.1016/j.jaad.2005.11.1079. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Cohen AD, David M, Hodak E, Chodik G, Viner A, Kremer E, Heymann A. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: A case-control study. J Am Acad Dermatol. 2007;56:629–634. doi: 10.1016/j.jaad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein. CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90:4812–4821. [PubMed] [Google Scholar]
- Xu K, Geczy CL. IFN-gamma and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. J Immunol. 2000;164:4916–4923. doi: 10.4049/jimmunol.164.9.4916. [DOI] [PubMed] [Google Scholar]
- Eue I, Langer C, Eckardstein A, Sorg C. Myeloid related protein (MRP) 14 expressing monocytes infiltrate atherosclerotic lesions of ApoE null mice. Atherosclerosis. 2000;151:593–597. doi: 10.1016/s0021-9150(00)00476-7. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, van de Kerkhof PC, Traupe H, de Jongh G, den Heijer M, Reis A, Armour JA, Schalkwijk J. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peters T, Kess D, Sindrilaru A, Oreshkova T, Van Rooijen N, Stratis A, Renkl AC, Sunderkotter C, Wlaschek M, Haase I, Scharffetter-Kochanek K. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116:2105–2114. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci USA: 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Kopp T, Kieffer JD, Rot A, Strommer S, Stingl G, Kupper TS. Inflammatory skin disease in K14/p40 transgenic mice: evidence for interleukin-12-like activities of p40. J Invest Dermatol. 2001;117:618–626. doi: 10.1046/j.1523-1747.2001.01441.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.