Figure 1. Proposed mechanisms of actin assembly factors.
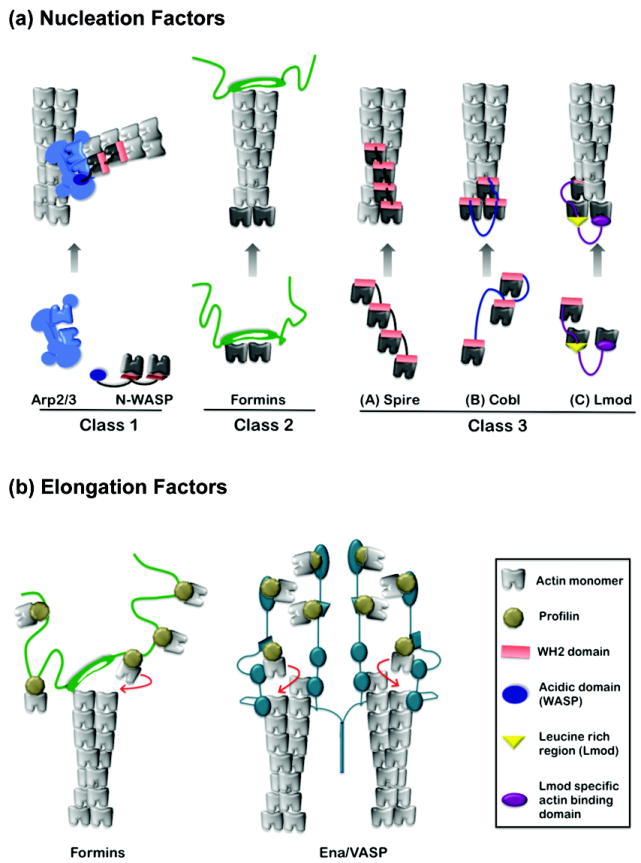
(a) Three classes of actin nucleators. Nucleator domains are displayed in color, actin subunits used by nucleators to seed polymerization in black, and actin subunits polymerized from nuclei in grey. Class I: N-WASP uses its WH2 domain(s) to recruit actin monomers and its acidic (A) domain to bind to an actin-related protein subunit of Arp2/3 complex. This structure stabilized by N-WASp may mimic an actin trimer. Class II: formins are hypothesized to nucleate actin polymerization by stabilizing spontaneously formed actin dimers and/or trimers. Formins remain associated with the barbed end while permitting addition of actin subunits. Class III: Spire, Cobl and Lmod contain between one and four WH2 domains each, separated by intervening linker sequences of variable length. Their nucleation mechanisms are related, but each may generate an actin nucleus with distinct properties, stabilized by lateral and/or longitudinal contacts between subunits, and in some cases capped at one end. Note, in some respects N-WASp represents a specialized form of Class III nucleator, in which the third actin monomer-binding domain has been replaced with a domain that binds to actin-related proteins. (b) Actin elongation factors. Formins shield barbed end growth from capping proteins by using their dimeric FH2 domains to processively move with the filament end. Adjacent rope-like FH1 domains are used as “arms” to recruit profilin-actin complexes and ‘deliver’ them to the FH2-capped filament end for rapid addition. The elongation mechanism of Ena/VASP is not well understood. However, it tetramerizes, bundles filaments, and may engage multiple barbed ends simultaneously. Its ability to accelerate barbed end elongation could involve a relay or hand-off of actin monomers using multiple actin-binding domains (adapted from model in [19]).
