Figure 2. Physical complexes that spatially and temporally coordinate multiple actin assembly factors.
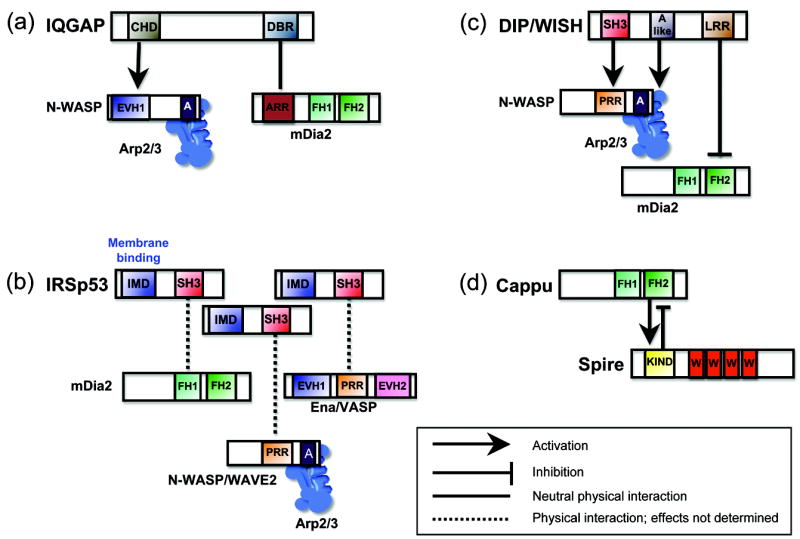
(a) IQGAP uses its calponin homology domain (CHD) to associate with the EVH1 domain of N-WASP, and its dia-binding region (DBR) to associate with the ankrin-rich repeats (ARR) of mDia2. (b) IRSp53 uses its IMD domain to bind and induce curvature of membranes and its SH3 domain to interact with the proline-rich region (PRR) of N-WASP and WAVE2, the PRR of Ena/VASP, and the proline-rich FH1 domain of mDia2. (c) DIP/WISH uses its SH3 domain to bind the PRR of N-WASP and an acidic (A-like) domain to bind Arp2/3 complex, leading to N-WASP-Arp2/3 activation, and its leucine-rich repeat (LRR) domain to directly inhibit FH2 domain of mDia2. (d) Spire uses its KIND domain to inhibit the FH2 domain of Cappuccino, while this same interaction enhances Spire activity.
