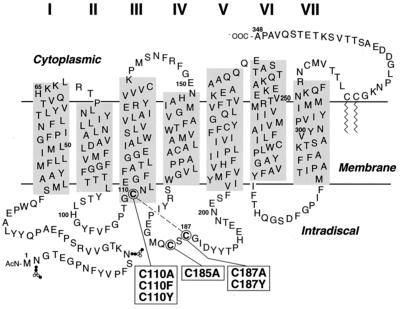
Figure 1.
A secondary-structure model of bovine rhodopsin showing the transmembrane domain with lengths of individual helices determined from electron microscopy by Unger et al. (23) and Baldwin et al. (24). The horizontal line depicting the membrane aqueous boundary on the cytoplasmic face is derived from EPR data (ref. 25 and unpublished work). The three intradiscal cysteines are circled. The mutants studied in this work are shown in boxes connected to the intradiscal cysteines. The dashed line between C110 and C187 indicates the disulfide bond in WT rhodopsin.