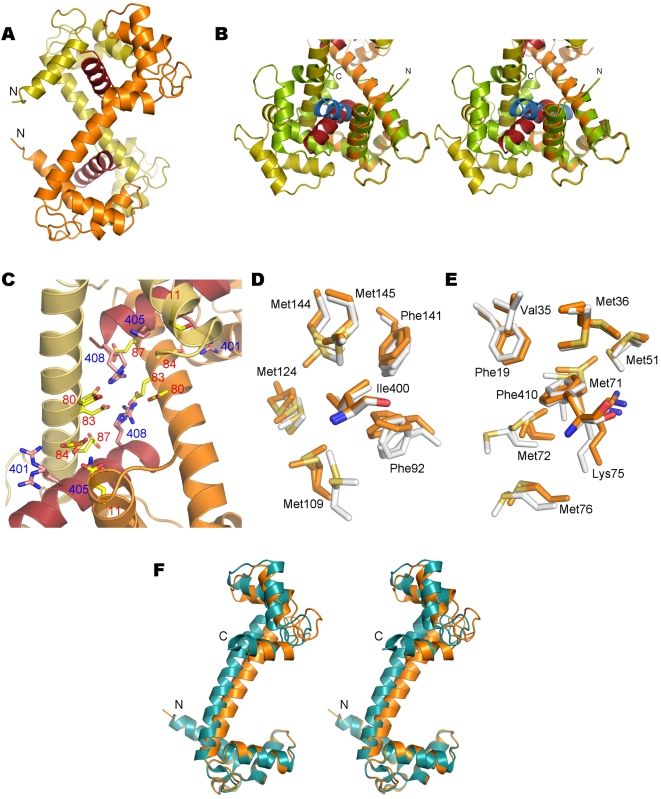
Figure 1. Crystal structure of the CaM-CnA-CBD complex.
A. Overall structure. The two peptides are shown in red and the two monomers of CaM in yellow and orange. The N termini of both CaM monomers point to the left. B. Comparison to a classical collapsed structure of a CaM-peptide complex (PDB entry 1WRZ). The superposition was carried out using the N-terminal lobe of CaM (to the right on this view). The colours for the CaM-CnA-CBD complex are as in A, and in the collapsed conformation, CaM is shown in green and the peptide in blue. The location of the hinge, related to the formation of the collapsed conformation, at the middle of the long linker helix is clearly visible. The N and C termini of the collapsed structure are labeled. C. The central part of the X-shaped complex contains a network of salt bridges. Most of the residues involved are mobile in the crystal and have been built in two conformations. The labels for acidic residues from CaM are in red and those for basic residues in the peptide in blue. D. The environment of the N-terminal anchoring residue Ile400 from the peptide in the C-terminal hydrophobic pocket of CaM. Note that all CaM residues forming the pocket are in double conformations. The structure is shown from two different monomers in the asymmetric unit of the structure (white and orange colours). E. The environment of the C-terminal anchoring residue Phe410 from the peptide in the N-terminal hydrophobic pocket of CaM. All 5 methionine residues in the pocket are disordered, and Phe410 is also in a double conformation. Colouring as in D. F. Comparison to the unliganded structure. The stereo view indicates that the structure of the CaM monomer in the complex (orange) is highly similar to that of unliganded CaM (blue).