Abstract
The microbes that accompany the etiologic agent of cholera, Vibrio cholerae, are only now being defined. In this study, spirochetes from the genus Brachyspira were identified at high titers in more than one third of cholera patients in Bangladesh. Spirochetosis should now be tracked in the setting of cholera outbreaks.
Keywords: Spirochetosis, spirochete, cholera, Vibrio cholerae, Brachyspira, coinfection, secretory diarrhea, Bangladesh, dispatch
Cholera in humans results in profuse, watery diarrhea that can lead to severe dehydration and death (1). The infection begins with ingestion of Vibrio cholerae from contaminated water, after which expression of cholera toxin induces a fluid loss that may reach 1 liter/hour. Cholera is an underreported disease in the developing world, and the true incidence may reach 2 million cases/year (2).
Because anecdotal evidence has indicated frequent cholera and intestinal spirochetosis coinfection in Bangladesh, we studied both diseases in this study. Intestinal spirochetosis has negative effects on domestic swine and poultry industries (3). In humans, 1%–64% of colonic specimens demonstrate intestinal spirochetosis, with the highest prevalence in developing countries and immunocompromised populations (4). The etiologic agents of intestinal spirochetosis are members of the genus Brachyspira (formerly Serpulina and Treponema). B. pilosicoli isolated from humans cause disease in pigs and in chicken models of infection (5,6). B. aalborgi isolated from humans do not colonize animals (7). Surveillance of intestinal spirochetosis requires molecular tools because culture has limited sensitivity caused by the fastidious nature of Brachyspira spp. (8). Patients with symptomatic intestinal spirochetosis have chronic diarrhea or soft feces (4,9,10); these clinical signs may resolve with antimicrobial drug therapy (9). Histologic analysis of intestinal biopsy specimens showed densely packed spirochetes attached by 1 end to colonic epithelium, forming a false brush border (11). Invasion of colonic epithelial cells and bleeding may occur (4,12). Virulence mechanisms remain poorly understood. Research on a vaccine to protect pigs against intestinal spirochetosis has begun. However, there is no vaccine for protection against intestinal spirochetosis in humans.
Co-infection of cholera patients with additional pathogens has focused on enterotoxigenic Escherichia coli (ETEC); 13% of cholera patients in Bangladesh are co-infected with ETEC (13). The potential for pathogenic synergy between V. cholerae and other pathogens has been proposed but not investigated.
The Study
We defined the frequent presence of spirochetes in feces of patients with cholera. A 3-step method was used to establish the distribution of spirochetes in rice-water stool: 1) the presence of spirochetes in rice-water stool was determined by using dark-field microscopy; 2) the diversity of Brachyspira spp. within a subset of these patients was quantified by 16S rDNA analysis; and 3) the genetic diversity within the most abundant species was determined by analysis of NADH oxidase (nox).
Rice-water stool samples were collected from symptomatic cholera patients (>15 years of age with no history of antimicrobial drug therapy) during the spring cholera outbreak of 2006 in Dhaka, Bangladesh, at the International Centre for Diarrheal Disease Research, Bangladesh, as part of a larger study (14). Samples were examined by dark-field microscopy for V. cholerae and other bacteria, and the presence of V. cholerae, lytic vibriophage, and ETEC was determined by using standard methods (14). Samples were preserved at 4°C in formalin, or cell pellets were resuspended in phosphate-buffered saline and 15% glycerol and stored at –80°C. Analysis showed that 36% (23/64) of samples that were positive for only V. cholerae also harbored spirochetes (Technical Appendix, panels A–F). Spirochetes were also found in 4/11 and 5/15 samples that harbored ETEC alone or both ETEC and V. cholerae, respectively. When we removed ETEC-positive samples as potential confounders, the presence of spirochetes was independent of lytic vibriophage in V. cholerae–positive stool samples (Technical Appendix, panel G), which is in contrast to the documented trend concerning non–V. cholerae bacteria in rice-water stool (14). The ratio of spirochetes to V. cholerae was ≈1 and independent of lytic vibriophage (Technical Appendix, panel H).
In the second step, 10 samples were chosen for molecular analysis on the basis of a large amount of spirochetes. Samples were heated (99°C for 10 min), and standard PCR for a diagnostic segment of the 16S rDNA gene (15) was performed by using genus Brachyspira–specific primers (5′-GTCTTAAGCATGCAAGTC-3′ and 5′-AACAGGCTAATAGGCCG-3′). Products were cloned and sequenced bidirectionally (GenBank accession nos. FJ599620–FJ599639). B. pilosicoli was the most common species, found in all 10 samples (Technical Appendix, panel I). B. aalborgi was the second most common species (7 samples). A second set of panspecific 16S rDNA degenerate primers (5′-GTTTGATYCTGGCTYAGARCKAACG-3′ and 5′-CCSSTACGGMTACCTTGTTACG-3′) confirmed the presence of Brachyspira spp. and suggested that spirochetes of other genera were not present. The added resolution of nox analysis also identified B. hyodysenteriae at lower abundance.
Culture, purification, and microscopy of B. pilosicoli from glycerol stocks was used to cross-validate the molecular approach. Standard fastidious anaerobe agar (FAA) supplemented with bovine blood (10%), spectinomycin (400 μg/mL), and polymyxin B (5 μg/mL) was determined empirically to be the optimal medium for isolation of spirochetes. Dilutions of glycerol stocks from each patient were plated on FAA agar; single colonies were best obtained with an FAA overlay. Plates were incubated for 21 days at 37°C in an atmosphere of 94% H2 and 6% CO2. Most colonies gave rise to sigmoidal spiral cell morphotypes similar to the morphotype of the American Type Culture Collection (ATCC) (Manassas, VA, USA) control strain for B. pilosicoli. Additional controls were ATCC B. hyodysenteriae, Helicobacter pylori, and Borrelia burgdorferi. Subsequent molecular analysis of patient isolates confirmed them as B. pilosicoli. Consistent with previous studies, B. aalborgi was not cultured from patient samples.
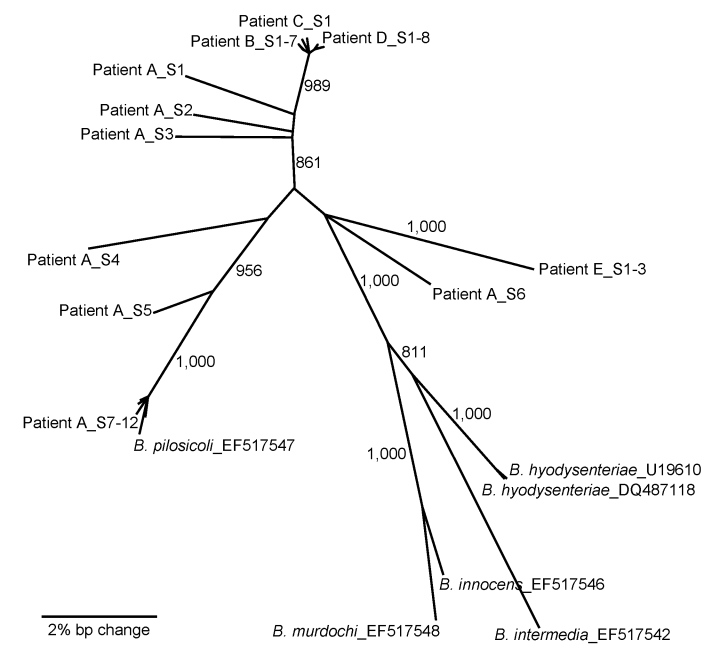
In the third step, 5 samples were analyzed by using nox sequence comparisons that yield higher phylogenetic resolution. DNA was extracted and a nox segment was amplified by using degenerate primers (5′-GCYGGHACATGGGCDGCAAAAAC-3′ and 5′-CAAATACRCAAATAGCRTTAG-3′). Products were cloned and sequenced bidirectionally (GenBank accession nos. FJ599589–FJ599619). B. pilosicoli was the most common nox sequence found. A phylogenetic tree (Figure) demonstrated that individual patients harbored clonal lines of B. pilosicoli (patients B, D, and E) or more diverse strains (patient A). Overall, B. pilosicoli strains found in cholera patients were extremely diverse relative to the known outgroup species, which indicates the potential for detection of new species related to intestinal spirochetosis.
Figure.
Neighbor-joining (NJ) phylogeny of NADH oxidase (nox) sequences of Brachyspira pilosicoli from 5 cholera patients (A–E). The nox sequences were PCR amplified, cloned, and sequenced from each patient (individual clones are appended _SX). Published sequences from known species are included for reference. NJ analysis was performed by using an NJ model and 1,000 bootstraps. Bootstrap values >800 are presented next to nodes. The scale bar indicates a 2% bp change (contiguous sequence ≈990 bp).
Conclusions
It has been casually observed for a century that stool from cholera patients harbors spirochete-like bacteria. We now define the major agents present as B. pilosicoli and B. aalborgi. More than one third of the cholera patients had spirochetes in their stools at densities equal to those of V. cholerae. The pathophysiology of intestinal spirochetosis in this setting and its relevance to human health remain unknown. Epidemiologic analysis of intestinal spirochetosis has so far relied on retrospective studies of colonic tissue collected for reasons secondary to the disease (4). We recommend a community-based prospective study of stool samples from healthy persons and patients with diarrhea by using the techniques described herein; animal reservoirs should be identified as a potential point of control. In the context of V. cholerae infection, we hypothesize that spirochetes may be present before V. cholerae infection and exacerbate the already devastating clinical course of cholera.
Supplementary Material
Presence of spirochetes (Spiro) in cholera stools. Representative images of planktonic and aggregated spirochetes. Vibrio cholerae (VC) were labeled with a fluorescein isothiocyanate–conjugated monoclonal antibody (Ab) to O1 lipopolysaccharide (panels A and D), and nucleic acid was labeled with 4′,6-diamidino-2-phenylindole (DAPI) (panels B and E), which showed sigmoidal morphology with tapered ends (4–8 µm) indicative of spirochetes. The single curve or W shape is indicative of Brachyspira pilosicoli. Merged images (panels C and F) showed V. cholerae and spirochetes. The aggregate (panel F) was also stained for mucus (blue indicates wheat germ agglutinin lectin) and showed that only a small portion of the aggregate contained mucus. Panel G shows spirochetes counted by direct count microscopy in rice-water stool samples relative to the presence or absence of lytic vibriophage. Panel H shows the ratio of spirochetes to V. cholerae by direct count microscopy relative to the presence or absence of vibriophage. Panel I shows the percentage of spirochetes that were B. pilosicoli as determined by using partial sequence analysis of 16S rDNA. PCR amplified products from isolated DNA were cloned and sequenced. Those clones that did not harbor B. pilosicoli sequence harbored B. aalborgi sequence. NEG, negative; POS, positive; Tot, total. Horizontal bars are median values with interquartile range as whiskers; medians were not significantly different (p>0.05, by Mann-Whitney U test); Scale bars = 10 µm.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant AI055058 to A.C. E.J.N. is a recipient of the Fogarty/Ellison Fellowship in Global Health awarded by the Fogarty International Center at NIH (D43 TW005572). A.C. is an investigator of the Howard Hughes Medical Institute.
Biography
Dr Nelson is a medical student at Tufts University School of Medicine. His research interests include relationships between host, pathogen, and bacteriophages in cholera.
Footnotes
Suggested citation for this article: Nelson EJ, Tanudra A, Chowdhury A, Kane AV, Qadri F, Calderwood SB, et al. High prevalence of spirochetosis in cholera patients, Bangladesh. Emerg Infect Dis [serial on the Internet]. 2009 Apr [date cited]. Available from http://www.cdc.gov/EID/content/15/4/571.htm
References
- 1.Wachsmuth IK, Blake PA, Olsvik Ø. Vibrio cholerae and cholera: molecular to global perspectives: Washington: American Society for Microbiology Press; 1994. [Google Scholar]
- 2.Sack DA, Sack RB, Chaignat CL. Getting serious about cholera. N Engl J Med. 2006;355:649–51. 10.1056/NEJMp068144 [DOI] [PubMed] [Google Scholar]
- 3.Duhamel GE. Comparative pathology and pathogenesis of naturally acquired and experimentally induced colonic spirochetosis. Anim Health Res Rev. 2001;2:3–17. [PubMed] [Google Scholar]
- 4.Korner M, Gebbers JO. Clinical significance of human intestinal spirochetosis: a morphologic approach. Infection. 2003;31:341–9. [DOI] [PubMed] [Google Scholar]
- 5.Trott DJ, Huxtable CR, Hampson DJ. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect Immun. 1996;64:4648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trott DJ, McLaren AJ, Hampson DJ. Pathogenicity of human and porcine intestinal spirochetes in one-day-old specific-pathogen-free chicks: an animal model of intestinal spirochetosis. Infect Immun. 1995;63:3705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trott DJ, Hampson DJ. Evaluation of day-old specific pathogen-free chicks as an experimental model for pathogenicity testing of intestinal spirochaete species. J Comp Pathol. 1998;118:365–81. 10.1016/S0021-9975(07)80012-0 [DOI] [PubMed] [Google Scholar]
- 8.Mikosza AS, La T, Margawani KR, Brooke CJ, Hampson DJ. PCR detection of Brachyspira aalborgi and Brachyspira pilosicoli in human faeces. FEMS Microbiol Lett. 2001;197:167–70. 10.1111/j.1574-6968.2001.tb10599.x [DOI] [PubMed] [Google Scholar]
- 9.Esteve M, Salas A, Fernandez-Banares F, Lloreta J, Marine M, Gonzalez CI, et al. Intestinal spirochetosis and chronic watery diarrhea: clinical and histological response to treatment and long-term follow up. J Gastroenterol Hepatol. 2006;21:1326–33. 10.1111/j.1440-1746.2006.04150.x [DOI] [PubMed] [Google Scholar]
- 10.Margawani KR, Robertson ID, Brooke CJ, Hampson DJ. Prevalence, risk factors and molecular epidemiology of Brachyspira pilosicoli in humans on the island of Bali, Indonesia. J Med Microbiol. 2004;53:325–32. 10.1099/jmm.0.05415-0 [DOI] [PubMed] [Google Scholar]
- 11.Guzman G, Weisenberg E. Intestinal spirochetosis. Arch Pathol Lab Med. 2004;128:1188. [DOI] [PubMed] [Google Scholar]
- 12.Gebbers JO, Marder HP. Unusual in vitro formation of cyst-like structures associated with human intestinal spirochaetosis. Eur J Clin Microbiol Infect Dis. 1989;8:302–6. 10.1007/BF01963456 [DOI] [PubMed] [Google Scholar]
- 13.Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, et al. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson EJ, Chowdhury A, Harris JB, Begum YA, Chowdhury F, Khan AI, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc Natl Acad Sci U S A. 2007;104:19091–6. 10.1073/pnas.0706352104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraaz W, Pettersson B, Thunberg U, Engstrand L, Fellstrom C. Brachyspira aalborgi infection diagnosed by culture and 16S ribosomal DNA sequencing using human colonic biopsy specimens. J Clin Microbiol. 2000;38:3555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Presence of spirochetes (Spiro) in cholera stools. Representative images of planktonic and aggregated spirochetes. Vibrio cholerae (VC) were labeled with a fluorescein isothiocyanate–conjugated monoclonal antibody (Ab) to O1 lipopolysaccharide (panels A and D), and nucleic acid was labeled with 4′,6-diamidino-2-phenylindole (DAPI) (panels B and E), which showed sigmoidal morphology with tapered ends (4–8 µm) indicative of spirochetes. The single curve or W shape is indicative of Brachyspira pilosicoli. Merged images (panels C and F) showed V. cholerae and spirochetes. The aggregate (panel F) was also stained for mucus (blue indicates wheat germ agglutinin lectin) and showed that only a small portion of the aggregate contained mucus. Panel G shows spirochetes counted by direct count microscopy in rice-water stool samples relative to the presence or absence of lytic vibriophage. Panel H shows the ratio of spirochetes to V. cholerae by direct count microscopy relative to the presence or absence of vibriophage. Panel I shows the percentage of spirochetes that were B. pilosicoli as determined by using partial sequence analysis of 16S rDNA. PCR amplified products from isolated DNA were cloned and sequenced. Those clones that did not harbor B. pilosicoli sequence harbored B. aalborgi sequence. NEG, negative; POS, positive; Tot, total. Horizontal bars are median values with interquartile range as whiskers; medians were not significantly different (p>0.05, by Mann-Whitney U test); Scale bars = 10 µm.