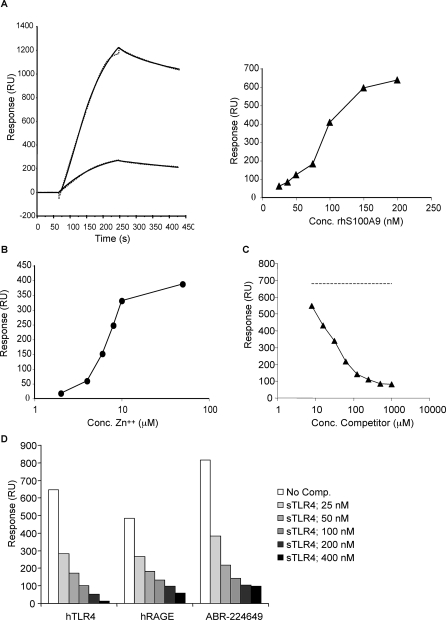
Figure 3. Interaction of Human S100A9 with Human TLR4/MD2.
(A) Left: binding of 100 nM S100A9 (upper) and S100A8/9 (lower) to immobilized TLR4/MD2 (coupling density ∼3,000 RU) represented as sensorgrams (solid lines). Best fit of curves was obtained with 1:1 model with mass transfer (dotted lines) with KD values of 2.1 and 3.8 nM and maximum responses at 1,270 and 280 RU, respectively. Right: half-maximal binding at approximately 77 nM was obtained when plotting responses at late association phase against concentration (Conc.) of S100A9.
(B) Influence of Zn2+ on S100A9 binding to hTLR4/MD-2 at a fixed concentration of Ca2+ (1 mM). Half-maximal binding was obtained at 6.4 μM Zn2+.
(C) Displacement curve showing inhibition of S100A9 binding to immobilised hTLR4/MD2 at 100 nM with the competitor (ABR-215757) added in a concentration range from 7.81 to 1,000 μM. Direct binding of ABR-215757 to hTLR4/MD2 was negligible (unpublished data). In this experiment, an IC50 value of 23 μM (r 2 = 0.998) was calculated after fit of data to a one-site competition model. Response for S100A9 in the absence of compound is indicated by the dashed line.
(D) Blocking of human S100A9 binding to immobilized TLR4/MD2, RAGE and Q compound by human TLR4/MD2 complex in solution. Comp, competitor.