Fig. 2.
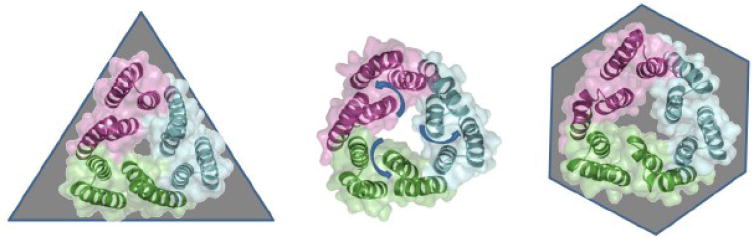
Views of the periplasmic aperture of the currently available TolC trimer structures reveal a possible transition to a fully open state. Structures of the closed state (1EK9; left), and partially open mutant structures 2VDE (center) and 2VDD (right) reveal a rearrangement of the periplasmic tip helices consistent with the proposed “aperture opening” mechanism. In the initial state (left) the aperture is maintained closed by inter-protomer salt-bridges, which are thought to become destabilized and released upon the interaction of the OMF with the RND. The following transition is also associated with a breakage of the central three-fold symmetry of the trimer and relaxation of the gating helical pairs (arrows) resulting in a roughly hexagonal arrangement (right).
