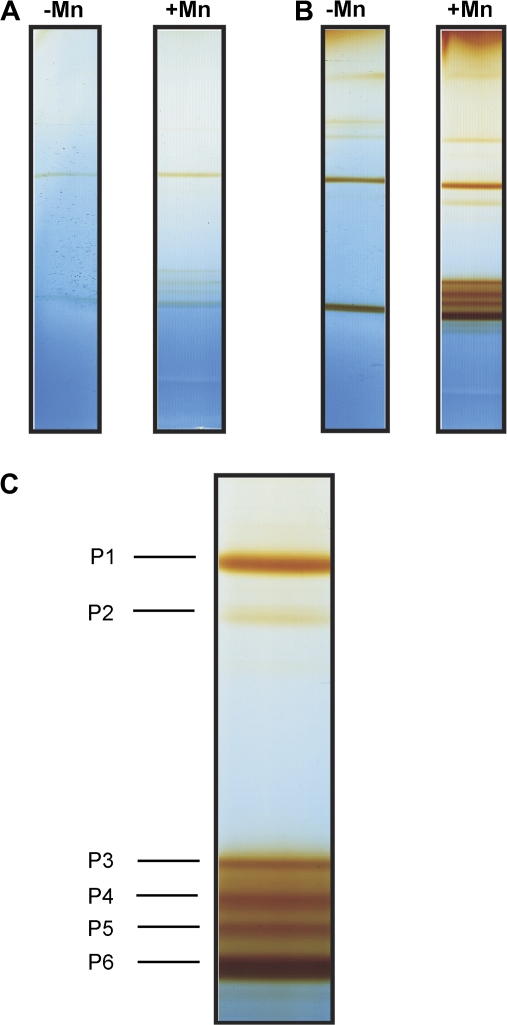
Fig. 3.
AWFNaCl-proteins of the second oldest trifoliate leaf of the Mn-sensitive cultivar TVu 91 stained for (A) NADH-peroxidase and (B) guaiacol-peroxidase activity after separation by BN-PAGE. After preculture with 0.2 μM Mn (–Mn) for 14 d, plants received 50 μM (+Mn) Mn for 6 d. Fifty μl of concentrated AWFNaCl containing ionically bound proteins (–Mn 60 μg, +Mn 112 μg) were loaded onto the gels. Proteins were NBT-stained for NADH-peroxidase (A) at pH 5.0 with 16 mM MnCl2, 1.66 mM p-coumaric acid, 0.625 mg ml−1 NBT, and 0.22 mM NADH. For guaiacol-peroxidase, proteins were stained (B) in 18 mM guaiacol (in 9 mM Na2HPO4) and 0.03% H2O2 at pH 6.0. Close up (C) shows marked isoenzymes (P1, P3, P5, P6) that were chosen for elution and further characterzation of pH optima and substrate specificity.