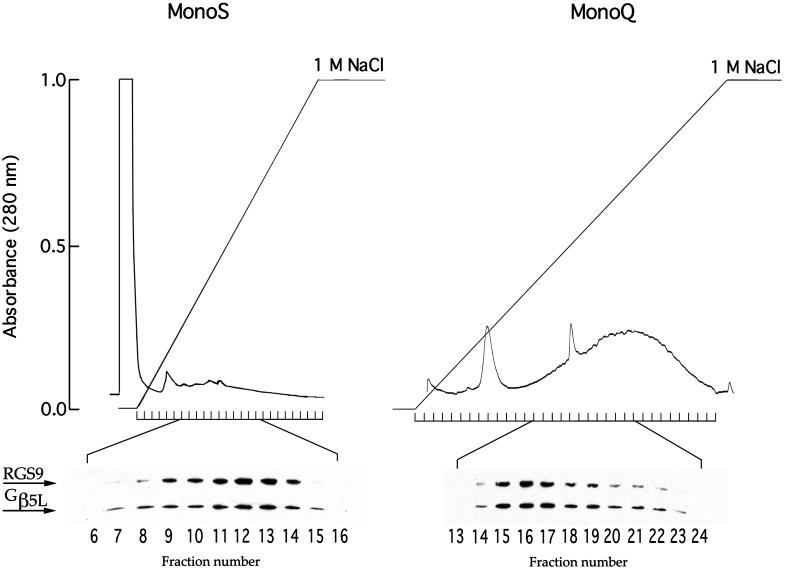
Figure 2.
Comigration of RGS9 and Gβ5L during cation-exchange and anion-exchange chromatography. Washed ROS membranes (containing 3 mg rhodopsin) were solubilized in 500 μl of 2% lauryl sucrose either in 20 mM Hepes-KOH (pH 6.0) with 2 mM MgCl2 (MonoS) or in 50 mM Tris⋅HCl (pH 7.8) with 2 mM MgCl2 (MonoQ). The columns were equilibrated by the corresponding buffers containing 0.5% lauryl sucrose. A 0–1 M gradient of NaCl was used to elute the bound proteins. The flow rate was 1 ml/min; the fraction size was 1 ml. Western blots of the chromatography fractions were probed by a mixture of rabbit anti-RGS9c and anti-NTS antibodies against Gβ5 at dilutions yielding similar intensities of immunostaining. No GAP activity in corresponding fractions was present in these cases, consistent with previous reports that this activity is extremely unstable in detergent solutions (32).