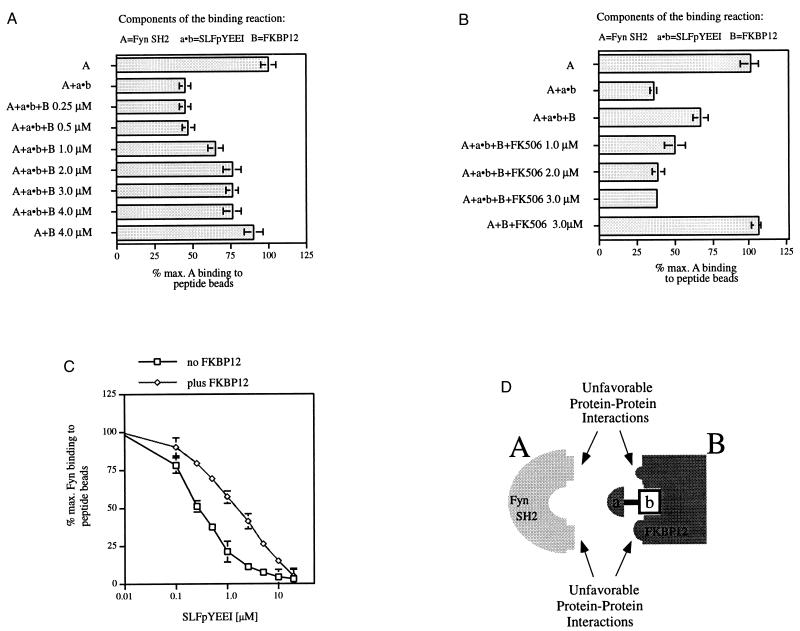
Figure 4.
Fyn SH2 domain binding to SLFpYEEI in a complex with FKBP12. (A–C) In 100 μl of binding buffer, peptide beads (7.5 μl) were incubated with the 32P-labeled Fyn SH2 domain (200 nM, 18,000 cpm) and the molecules stated below. After a 2-hr incubation time, the beads were centrifuged and the radioactive Fyn SH2 domain bound was quantitated. (A) Addition of SLFpYEEI (1.0 μM) and increasing concentrations of FKBP12 (0.25–4.0 μM). (B) Addition of SLFpYEEI (1.0 μM), FKBP12 (2.0 μM), and increasing concentrations of FK506 (1.0–3.0 μM). The maximal radioactivity associated with the beads (=100%) was 12,583 cpm for A and 12,187 cpm for B. (C) Competition binding curves: 32P-labeled Fyn SH2 domain (200 nM, 13,000 cpm) was incubated without or with FKBP12 (20 μM) in 100 μl of binding buffer. Increasing concentrations of FKpYEEI (0.1–20.0 μM) were added to the binding reactions. The maximal radioactivity associated with the beads was 9,284 cpm (=100%) for the 32P-labeled Fyn SH2 domain alone and 10,492 cpm (=100%) for the 32P-labeled Fyn SH2 domain in the presence of FKBP12. All data points were taken in triplicate, and the average was plotted. (Bars = SE.) (D) Model for unfavorable, destabilizing protein–protein interactions.