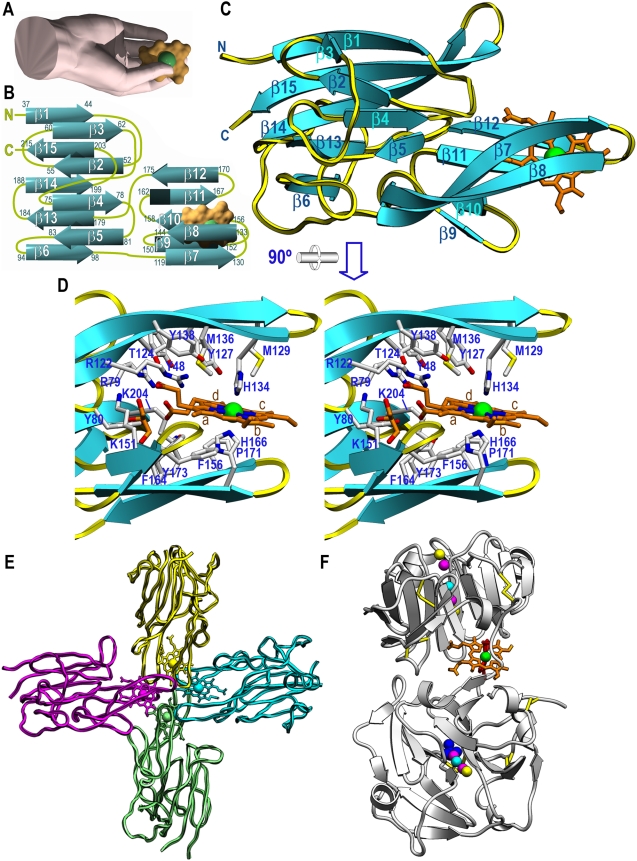
Figure 4. Structure of holo-HmuY.
(A) The protein mimics a right hand, whose thumb and fingers trap the heme co-factor (surface model in orange with an inserted green sphere for the iron ion). (B) Topology scheme of HmuY, which consists of 15 β-strands, each labeled with the protein residues it spans. The heme group is shown as in (A). (C) Richardson plot of holo-HmuY Glu35-Lys216 with the bound heme as an orange stick model and an inserted sphere for the iron. The view was chosen to match (A). (D) Close-up view in stereo of (C) after a horizontal 90° rotation. Protein residue side chains engaged in shaping the heme-binding cavity and in interactions with the co-factor are shown and labeled. The four pyrrole rings of the protoporphyrin IX moiety are also labeled (a–d). (E) Tetrameric quaternary arrangement of holo-HmuY. Each constituting heme/HmuY complex is shown in one color. (F) Richardson plot of rabbit serum hemopexin (PDB access code 1QHU; [32]). The two hemopexin-like β-propeller domains contain central channels to bind ions (colored spheres), and the heme-binding site is at the domain intersection.