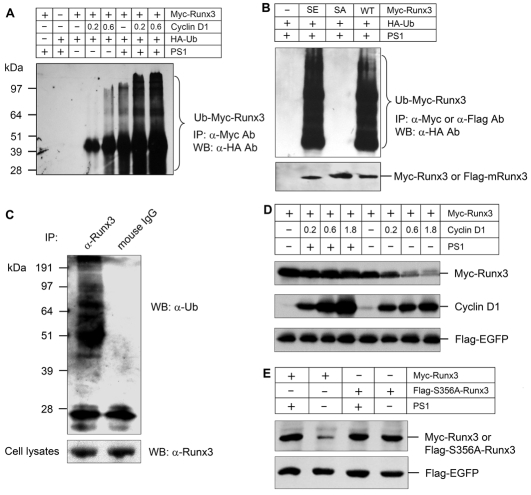
Fig. 4.
Cyclin D1 induces ubiquitylation and proteasome degradation of Runx3. (A) Myc-Runx3 and HA-ubiquitin expression plasmids were cotransfected with different amounts of cyclin D1 expression plasmid (0.2 and 0.6 μg/6-cm culture dish) into COS cells in the presence or absence of PS1 (10 μM for 4 hour incubation). Cyclin D1 induced Runx3 ubiquitylation in a dose-dependent manner and this effect was further enhanced by the addition of PS1. (B) Myc-Runx3, FLAG-S356A-Runx3 or FLAG-S356E-Runx3 was cotransfected with HA-ubiquitin plasmid into COS cells in the presence of PS1 (10 μM, 4 hours incubation). Runx3 ubiquitylation was detected in the WT Runx3 and S356E-Runx3 groups but not in the S356A-Runx3 group. (C) To further determine the ubiquitylation of endogenous Runx3, chondrogenic RCJ3.1C5.18 cells were treated without or with MG132 (10 μM, 4 hour incubation) before cell lysates were collected. Immunoprecipitation was performed using the anti-Runx3 antibody followed by western blotting using the anti-ubiquitin antibody. Ubiquitylation of endogenous Runx3 was detected in the presence of MG132 in RCJ3.1C5.18 cells. (D) Myc-Runx3 expression plasmid was cotransfected with different amounts of cyclin D1 expression plasmid (0.2, 0.6 and 1.8 μg/dish) into COS cells. Cells were treated with PS1 (10 μM) for 4 hours after transfection. Cyclin D1 induced a dose-dependent degradation of Runx3 and treatment with PS1 completely reversed cyclin-D1-induced Runx3 degradation. (E) WT and mutant Runx3 (S356A) expression plasmids were transfected into COS cells. Cells were treated with PS1 (10 μM, 4 hour incubation) after transfection. The addition of PS1 increased the level of WT Runx3 protein but had not effect on mutant Runx3 (S356A) protein.