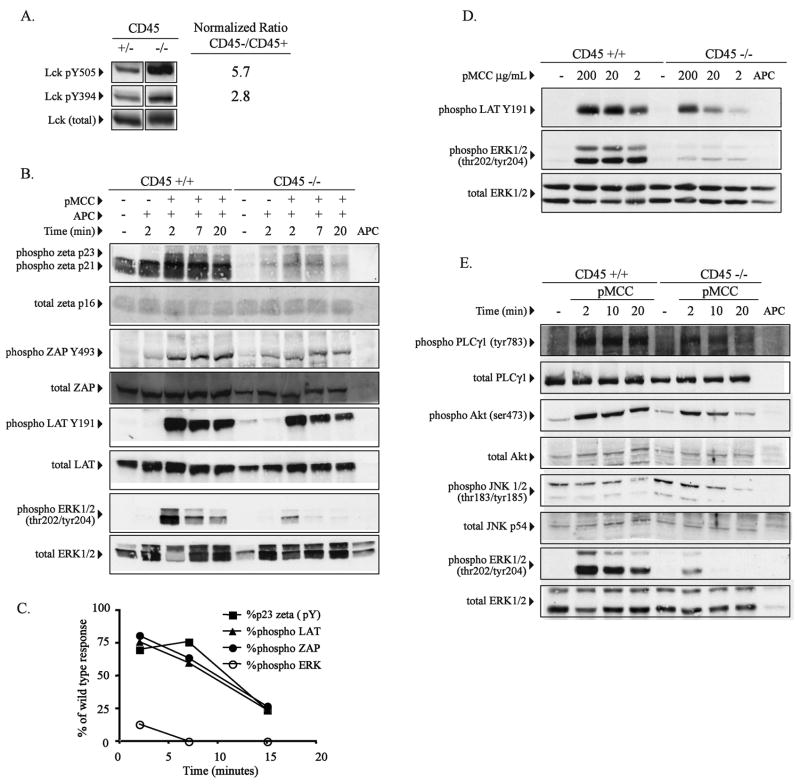
Figure 4.
ERK specific phosphorylation defect in double positive CD45 deficient thymocytes following stimulation with peptide. (A) Purified CD4+CD8+ double positive (DP) thymocytes from CD45+/+ or CD45−/− AND TCR transgenic mice were analyzed for Lck tyrosine phosphorylation at Y505 and Y394 using phosphospecific antibodies. Relative degree of phosphorylation at each site was quantified by densitometry after normalization for total Lck. The fold increase in tyrosine phosphorylation in CD45 deficient cells is indicated. (B) Purified DP thymocytes from CD45+/+ or CD45−/− AND TCR transgenic mice were stimulated with peptide pulsed APC (50 μg/ml, pMCC) as described in Figure 3 for the indicated time periods and analyzed for specific phosphosphorylation of TCR-zeta, ZAP70, LAT and ERK by western blot using the indicated phospho-specific antibodies. TCRzeta phosphorylation was detected using a pan-specfic phosphotyrosine antibody. The blots were then stripped and probed for total protein where indicated. (C) Densitometric analysis of the data in panel B is graphically represented following normalization for T cells using the total zeta western blot data. Data shown is the percent of the response of CD45 deficient cells compared to control CD45+ cells. (D) Purified DP thymocytes from wild type or CD45 deficient AND TCR transgenic mice were stimulated with APC’s pulsed with the indicated doses specific peptide for 5 minutes and phosphorylation of LAT and ERK1/2 was detected using the indicated phosphospecific antibodies. (E) As in panel B, purified DP thymocytes from wild type or CD45 deficient AND TCR transgenic mice were stimulated with peptide-pulsed APC for the indicated periods of time and activation of PLC-γ1, AKT, JNK1/2, and ERK1/2 was detected using the indicated phosphospecific antibodies. The data shown in each panel are representative of at least two independent experiments for each molecule. Data within each panel is derived from the same set of cell lysates.