Abstract
Aims
Severe heart failure (HF) is associated with cachexia; this is often reversed post cardiac transplantation (HTx) with frequent development of obesity. Growth hormone (GH) resistance is common in HF and may contribute to cachexia. Whether GH resistance resolves post HTx is unknown. We aimed to confirm that HF is associated with GH resistance and to test the hypothesis that GH resistance resolves post HTx.
Methods and results
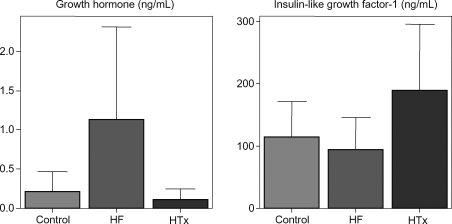
We measured GH, insulin-like growth factor-1 (IGF-1), and body composition in 10 HF patients awaiting HTx, in 18 patients 11 ± 8 months post HTx, and seven controls. Body mass index was 23.5 ± 3.2 in HF patients and 29.3 ± 5.7 post HTx. HTx patients had gained 14 ± 8 kg since HTx. GH was elevated in HF (control: 0.21 ± 0.25; HF: 1.13 ± 1.19; HTx: 0.11 ± 0.13 ng/mL; P < 0.007), while IGF-1 was higher in HTx (control: 114 ± 57; HF: 94 ± 52; HTx: 190 ± 106 ng/mL; P < 0.02). HTx had higher total body and abdominal fat %.
Conclusion
GH resistance is present in severe HF and resolves post HTx. These findings should be confirmed through larger trials.
Keywords: Heart failure, Cachexia, Cardiac transplantation, Growth hormone, Insulin-like growth factor-1
Background and aims
Cardiac cachexia is well described in severe heart failure (HF).1–3 Cardiac transplantation (HTx) is associated with weight gain and the frequent development of obesity. Although this weight gain has been attributed to glucocorticoid therapy, it is unrelated to steroid dose and more dramatic than after other solid organ transplants, despite similar steroid regimens,4 suggesting that other mechanisms are responsible. Growth hormone (GH) is an anabolic hormone secreted from the anterior pituitary that acts via insulin-like growth factor-1 (IGF-1), leading to protein synthesis and cell growth in target tissues such as skeletal muscle. It has been suggested that HF is associated with acquired GH resistance, defined as elevated GH with normal or low IGF-1.5,6 The GH/IGF-1 axis after transplantation has not been studied.
The aims were (1) to confirm that HF is associated with GH resistance and (2) to test the hypothesis that GH resistance resolves post HTx.
Methods
We studied 10 HF patients in New York Heart Association functional class IV awaiting HTx, 18 patients 11 ± 8 months post HTx, and seven controls. Subjects were age- and sex-matched. HF and HTx patients were matched by aetiology, and controls were matched to HTx patients by body mass index (BMI; Table 1).
Table 1.
Clinical characteristics and hormonal levels
| Group | Control (n = 7) | HF (n = 10) | HTx (n = 18) | P overall | P control vs. HF | P control vs. HTx | P HF vs. HTx |
|---|---|---|---|---|---|---|---|
| Age (years) | 42 ± 14 | 54 ± 13 | 49 ± 18 | NS | NS | NS | NS |
| Gender, n (%) | |||||||
| Males | 5 (71%) | 8 (80%) | 15 (83%) | ||||
| Females | 2 (29%) | 2 (20%) | 3 (17%) | NS | NS | NS | NS |
| Aetiology | |||||||
| Ischaemic | 4 (40%) | 7 (39%) | |||||
| Dilated | 4 (40%) | 9 (50%) | |||||
| Valvular | 2 (20%) | 2 (11%) | NS | ||||
| Prednisone dose (mg) | 6 ± 4 | ||||||
| Weight gain since HTx (kg and %) | 14 ± 8 kg, 22 ± 18% | ||||||
| Time since HTx (months) | 11 ± 8 | ||||||
| BMI (kg/m2) | 27.8 ± 4.2 | 23.5 ± 3.2 | 29.3 ± 5.7 | 0.02 | 0.09 | NS | <0.005 |
| Fat % | 26 ± 7 | 23 ± 2 | 32 ± 8 | <0.05 | NS | <0.11 | 0.02 |
| Lean mass % | 74 ± 7 | 77 ± 2 | 68 ± 9 | 0.05 | NS | <0.11 | 0.02 |
| Abdominal fat % of total abdominal mass | 27 ± 8 | 23 ± 4 | 34 ± 7 | <0.006 | NS | 0.04 | <0.003 |
| Waist circumference (cm) | 99 ± 10 | 86 ± 4 | 106 ± 15 | <0.02 | <0.14 | NS | <0.006 |
| Peak VO2 (mL/kg/min) | 27 ± 9 | 11 ± 2 | 16 ± 5 | <0.001 | <0.001 | <0.001 | 0.12 |
| REE % predicted | 95 ± 11 | 122 ± 28 | 109 ± 26 | 0.09 | 0.03 | NS | NS |
| Quadriceps cross-sectional area (cm2) | 105 ± 20 | 74 ± 14 | 93 ± 15 | <0.006 | <0.002 | 0.10 | 0.02 |
| GH (ng/mL) | 0.21 ± 0.25 | 1.13 ± 1.19 | 0.11 ± 0.13 | <0.001 | <0.007 | NS | <0.001 |
| IGF-1 (ng/mL) | 114 ± 57 | 94 ± 52 | 190 ± 106 | <0.02 | NS | <0.06 | <0.008 |
NS denotes non-significant and >0.2. P-values between 0.05 and 0.2 are considered non-significant but are listed as they may reflect trends. Normal levels for insulin-like growth factor-1 using this assay (Nichols Institute Diagnostics) are as follows: age 25–39 years: 114–492 ng/mL; age 40–54 years: 90–360 ng/mL; age ≥55 years: 71–290 ng/mL. HF, heart failure; HTx, cardiac transplantation; BMI, body mass index; REE, resting energy expenditure.
Body composition was assessed using dual-energy X-ray absorptiometry (DEXA; QDR 4500 A Delphi W densitometer, Hologic Inc., Bedford, MA, USA). Waist circumference was measured in duplicate at the level of the umbilicus. Resting energy expenditure (REE) and peak exercise VO2 were determined using a metabolic cart (Medical Graphics, Minneapolis, MN, USA). Fasting blood samples were collected in chilled ethylene diamine tetraacetic acid and centrifuged at 3000 rpm for 15 min at 4°C. Plasma was stored at −70°C. GH was measured by a two-site immunoradiometric assay (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). IGF-1 was measured by radio immunoassay using a polyclonal rabbit antibody generated against human IGF-I (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). The protocol was approved by the institutional review board of Columbia University Medical Center, and written informed consent was obtained from all patients.
Non-continuous variables were compared using χ2 analysis. Continuous variables were compared by one-way analysis of variance. Correlations were tested with both Pearson's and Spearman's correlations. A P-value <0.05 was considered statistically significant. All results are reported as mean ± standard deviation.
Results
Clinical, body composition, and laboratory data are summarized in Table 1. HTx patients had gained 14 ± 8 kg (22 ± 18%) over 11 ± 8 months since HTx. The medical and immunosuppressive regimens were representative of end-stage HF and post-transplantation, respectively.
The HF patients had the lowest BMI, body fat %, abdominal fat as % of abdominal mass, waist circumference, and quadriceps cross-sectional area. The HTx patients had higher abdominal fat % and a trend towards higher total body fat %. Quadriceps cross-sectional area was larger post HTx but less than control. Together, these data suggest that weight gain after HTx is predominantly fat and located mainly in the abdominal region. Peak VO2 was dramatically impaired in HF and somewhat impaired in HTx, and REE was significantly higher in HF than in controls but remained elevated post HTx.
GH levels were higher in HF than in control or HTx. In contrast, IGF-1 was comparable between HF and control but higher in HTx (Table 1 and Figure 1). Neither GH nor IGF-1 correlated with BMI overall or in HF.
Figure 1.
Growth hormone and insulin-like growth factor-1 levels in controls, heart failure, and post-cardiac transplantation.
Conclusion
The weight gain observed post HTx in this study is consistent with that of previous reports.2,4 REE was elevated in HF, which may contribute to cachexia, but remained elevated post HTx, and is unlikely to explain the weight gain. Here, we suggest that resolution of GH resistance may be one mechanism contributing to weight gain post HTx.
Severe HF is associated with GH resistance, defined as elevated GH with normal or low IGF-1,5,6 which may explain why GH therapy in HF has been largely unsuccessful,6,7 despite promising data in animal models.8 The mechanisms are largely unknown.9 IGF-1 may protect against cardiac and skeletal muscle apoptosis and low levels may contribute to apoptosis in HF. Skeletal muscle expression of IGF-1 is reduced in HF10 despite normal or mildly depressed plasma IGF-1 levels.11 Angiotensin II activation causes muscle wasting by decreasing muscle IGF-1 levels12 and angiotensin-converting enzyme-inhibitors may increase circulating IGF-1.9
We confirm that GH resistance occurs in severe HF. Interestingly, GH resistance is thought to be present in cachectic HF only,5,6 whereas in this study, it did not correlate with BMI. These findings are consistent with the fact that resistance to GH therapy occurs also in the absence of cachexia.6
To our knowledge, this is the first study to demonstrate that GH resistance resolves post HTx. This was evidenced by a reversal of the GH–IGF-I relationship post HTx from higher GH, lower IGF-I pre HTx to lower GH and higher IGF-I post HTx. Interestingly, although IGF-1 was significantly higher post HTx than in HF and there was a trend towards being higher than in controls (P < 0.06), these levels were above the age-referenced upper limit of normal level in only two of 18 patients and it would be premature to conclude that IGF-1 ‘overshoots’ post HTx.
GH is a potent anabolic hormone, an effect mediated by IGF-I. GH also has important metabolic effects including promoting lipolysis of adipose tissue,9 a direct effect of GH, unrelated to IGF-1. It is possible that in HF, a state of GH resistance to IGF-I production, the low IGF-I contributes to the observed catabolic state and the higher GH promotes adipose tissue lipolysis contributing to the observed lower % body fat and abdominal fat as % of abdominal mass. Once GH resistance resolves post HTx and GH levels fall, adipose tissue lipolysis is decreased and fat gain occurs.
This study is limited by the small number of patients, by the absence of target tissue IGF-1 levels such as from skeletal muscle, and by the fact that it was cross-sectional rather than longitudinal. Nevertheless, the finding that GH resistance is present in HF and not post HTx provides a basis for future larger studies of the role of GH/IGF-1 and other anabolic hormones in HF and after transplantation.
Funding
This study was supported by Stockholms Läns Landsting, Astrid och David Hageléns Stiftelse, and Magnus Bergvalls Stiftelse, Stockholm, Sweden (in the form of grants to Dr L.H.L.) and by the Division of Research Resources, General Clinical Research Centers Program, National Institutes of Health (5 MO1 RR00645), Bethesda, MD, the Foundation for Cardiac Therapies (FACT Fund), New York, NY, and the Altman Fund, New York, NY (in the form of grants to Dr D.M.M.).
Conflict of interest: none declared.
References
- 1.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Knosalla C, Birks E, Loebe M, Davos CH, Tsang S, Negassa A, Yacoub M, Hetzer R, Coats AJ, Anker SD. Heart transplantation in heart failure: the prognostic importance of body mass index at time of surgery and subsequent weight changes. Eur J Heart Fail. 2007;9:839–844. doi: 10.1016/j.ejheart.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 4.Williams JJ, Lund LH, LaManca J, Kunavarapu C, Cohen DJ, Heshka S, Heymsfield SB, Mancini DM. Excessive weight gain in cardiac transplant recipients. J Heart Lung Transplant. 2006;25:36–41. doi: 10.1016/j.healun.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, Ranke MB, Poole-Wilson PA, Giustina A, Dietz R, Coats AJ. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–452. doi: 10.1016/s0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 7.Ibe W, Saraste A, Lindemann S, Bruder S, Buerke M, Darius H, Pulkki K, Voipio-Pulkki LM. Cardiomyocyte apoptosis is related to left ventricular dysfunction and remodelling in dilated cardiomyopathy, but is not affected by growth hormone treatment. Eur J Heart Fail. 2007;9:160–167. doi: 10.1016/j.ejheart.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Bollano E, Bergh CH, Kjellstrom C, Omerovic E, Kujacic V, Caidahl K, Bengtsson BA, Waagstein F, Isgaard J. Growth hormone alone or combined with metoprolol preserves cardiac function after myocardial infarction in rats. Eur J Heart Fail. 2001;3:651–660. doi: 10.1016/s1388-9842(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 9.Cicoira M, Kalra PR, Anker SD. Growth hormone resistance in chronic heart failure and its therapeutic implications. J Card Fail. 2003;9:219–226. doi: 10.1054/jcaf.2003.23. [DOI] [PubMed] [Google Scholar]
- 10.Hambrecht R, Schulze PC, Gielen S, Linke A, Mobius-Winkler S, Yu J, Kratzsch JJ, Baldauf G, Busse MW, Schubert A, Adams V, Schuler G. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39:1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 11.Frost RA, Lang CH. Alteration of somatotropic function by proinflammatory cytokines. J Anim Sci. 2004;82(Suppl. E):E100–E109. doi: 10.2527/2004.8213_supplE100x. [DOI] [PubMed] [Google Scholar]
- 12.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]