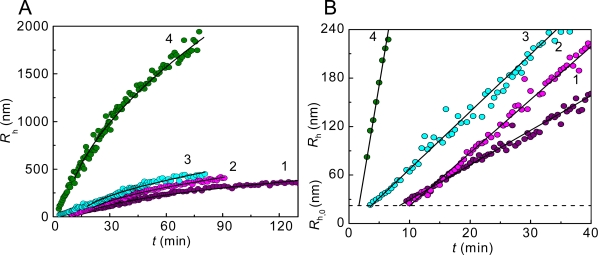
Figure 3.
Kinetics of thermal aggregation of GAPDH (0.4 mg/mL) at 55 °C. The dependences of the hydrodynamic radius of the protein aggregates (Rh) on time obtained at various concentrations of the enzyme: (1) 0.05, (2) 0.2, (3) 0.3 and (4) 0.4 mg/mL. (A) Full kinetic curves. The solid curves are calculated from Equation (8). (B) The initial parts of the dependences of Rh on time. The solid lines were calculated from Equation (9). The horizontal dotted line corresponds to the Rh,0 value (Rh,0 = 22 nm).