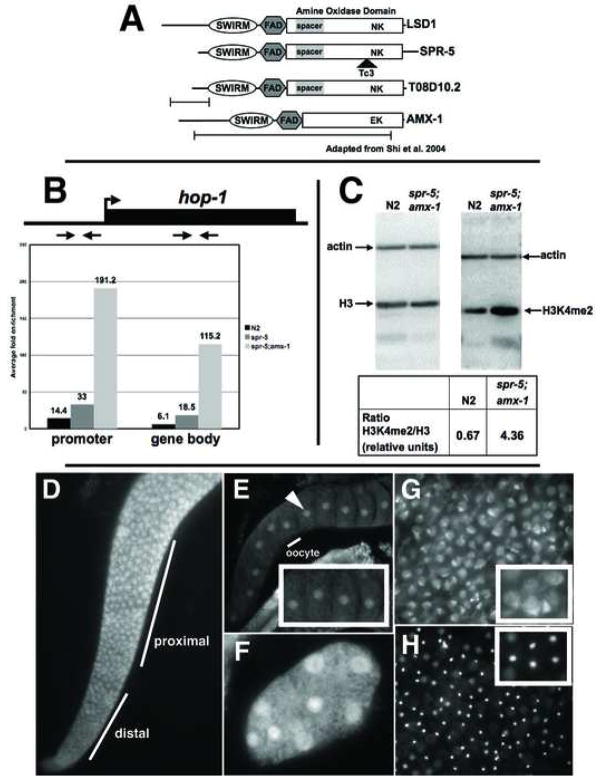
Figure 1.
spr-5 is a H3K4me2 demethylase. A, Alignment of the C. elegans LSD1/KDM1 orthologs with human LSD1/KDM1. The key catalytic residues (N660 and K661) are shown at their relative position within the amine oxidase domain and the location of the mutations are shown beneath each gene. B, ChIP of H3K4me2 at hop-1 in wild-type (N2), spr-5(by101) and spr-5;amx-1 mutants. The fold enrichment (of the percentage of input precipitated) in H3K4me2 Ab over no Ab, with the positions of the primer sets in the promoter and gene body indicated above. C, Western blots of wild-type (N2) and spr-5;amx-1 protein extracts probed with actin, histoneH3 and H3K4me2 (positions marked). The ratio of H3K4me2/histoneH3 normalized to actin is shown below. Immunofluorescence with SPR-5 N (D,G,H) and C terminal (E,F) Ab’s showing the adult gonad (D), oocytes (E), an early embryo (F), sperm (G), and the corresponding DAPI image of sperm (H). The inset in panels E,F and G are zoomed in on part of the panel. The identical staining pattern was observed with both N and C terminal Ab’s.