Fig. 1.
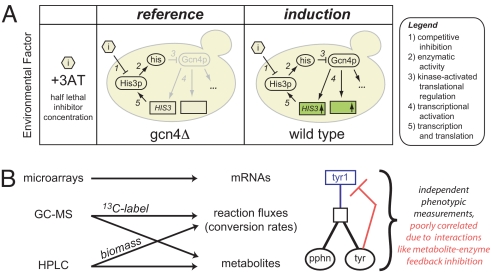
Experimental design and measurement strategy. (A) Analyzing the Gcn4p-dependent stress response in a controlled-growth chemostat environment. Wild-type S288C and an isogenic gcn4Δ derivative were cultured in a glucose-limited chemostat diluted at 0.10 h−1 (416 min per doubling) in unsupplemented YNB minimal media regulated to 5 +/− 0.1 pH. Titrated histidine biosynthesis inhibitor levels (10 and 0.1 mM, respectively, of 3-amino-1,2,4-triazole or 3AT) created histidine near starvation (Fig. S1), which causes Gcn4p translational activation and transcriptional activation of hundreds of targets in the wild type (+Gcn4p). Through this modulation of the inhibitor (3AT) concentration, both wild-type and gcn4Δ cultures were grown at the same specific growth rate (0.10 h−1) and achieved similar cell densities and production rates of ethanol and CO2 (Table S3). (B) Multitiered measurement strategy for analyzing large-scale network perturbations. We used microarrays, GC-MS, and HPLC to measure mRNAs, fluxes, and metabolites (see text and SI Text for further detail). These independent measurements give a broad view of the Gcn4p stress response and help to characterize network effects, such as metabolite interaction densities.