Abstract
Objective
To assess early circulatory status in very low birthweight (VLBW) infants with suspected intrauterine infections.
Patients
Thirteen VLBW infants who were diagnosed with prenatal infections because of raised serum IgM at birth (infectious group), and 39 infants matched for gestational age and birth weight (control group).
Methods
Echocardiographic assessments were performed consecutively from birth to day 28 in all VLBW infants. Left ventricular output (LVO) and left ventricular stroke volume (LVSV) were measured using Doppler echocardiography. Pulsed Doppler assessment of pulmonary artery pressure (PAP) was performed using the corrected ratio of the pulmonary artery acceleration time to the right ventricular ejection time (AT/RVET(c)). Blood flow in the superior mesenteric artery (SMA) was also evaluated by Doppler ultrasound.
Results
Mean LVO and LVSV were both significantly higher in the infectious group than in the control group at 12 hours (LVO; 188 v 154 ml/kg/min) and 72 hours (LVO; 216 v 173 ml/kg/min) of life. Pulsed Doppler assessment of PAP showed that mean AT/RVET(c) values were significantly lower in the infectious group than in the control group at 48 hours, 96 hours, day 14, and day 28. In the analysis of SMA flow velocities, both peak systolic velocities and time averaged velocities had decreased significantly in the infectious group compared with the control group at 24 hours, 36 hours, 96 hours, and day 28.
Conclusions
VLBW infants with suspected prenatal infection showed a unique circulation status, namely high cardiac output, latency of high PAP, and low organ flow.
Keywords: prenatal infection, cardiac output, pulmonary hypertension, organ blood flow, chronic lung disease
Prenatal intrauterine infections are known to cause premature labour and postnatal life threatening infections in both newborns and their mothers.1,2 These infections include maternal chorioamnionitis, which may induce increased release of various cytokines1,2,3,4 and may be a major risk factor for postnatal septicaemia and acute3,5,6 and chronic3,5,6 respiratory insufficiency. In extremely premature babies, prenatal infections are often associated with intractable chronic lung disease (CLD),5,6,7,8 which may affect the infant's quality of life in both the neonatal period and later years of life.
The premature cardiovascular system in preterm infants often seems unstable and fragile especially in the early neonatal period. The myocardium of preterm infants may be easily influenced by hypoxia and ischaemia, resulting in systemic and pulmonary hypoperfusion. In critically ill very low birthweight (VLBW) infants, reduced left ventricular function,9 decreased left ventricular output (LVO),10 and persistency of pulmonary hypertension11 have been reported. However, early longitudinal changes in LVO, pulmonary hypertension, and organ blood flow in unstable VLBW infants with suspected congenital infections have not been reported.
Immunoglobulin M (IgM) circulates in serum as a pentamer of disulphide linked immunoglobulin molecules. Although IgM is generated early in the immune response to antigen stimulation, its large size means that it cannot cross the placenta. Accordingly, high serum concentrations of IgM in a newborn infant at birth are regarded as evidence that the fetus can generate IgM prenatally when exposed to antigen stimulation in the womb.12 In preterm infants, raised concentrations of serum IgM have been shown to be significantly associated with both chorioamnionitis and chronic respiratory insufficiency.7,8 The present study was planned to evaluate early circulatory status by echocardiography in VLBW infants who were suspected of contracting intrauterine infection because of a significant increase in serum IgM at birth.
Patients and methods
A total of 206 VLBW infants weighing less than 1500 g were admitted to the neonatal intensive care unit at Kakogawa Municipal Hospital between September 2000 and December 2003. Of these, 43 small for gestational age infants, five infants with congenital abnormalities, 60 multiple births, and 10 infants who died within 48 hours of birth were excluded from the study; the remaining 104 VLBW infants were included in the analysis. A serum IgM concentration of >20 mg/dl at birth is usually regarded as clinically significant.12 Of the 104 VLBW infants, 13 had suspected intrauterine infection, with significantly raised serum IgM concentrations (designated the infectious group). Three controls matched for gestational age and birth weight were selected for each infant in the infectious group, and 39 infants were included in the analysis (control group). Gestational age was calculated from the mother's last menstrual day and confirmed by ultrasonography during pregnancy by obstetricians. Neonatal data were collected from nursery records. CLD was diagnosed in an infant with respiratory distress who required oxygen at 36 weeks corrected age. Septicaemia was suspected from clinical findings and proved by positive blood culture. The study was approved by the local ethics committee at Kakogawa Municipal Hospital.
All VLBW infants admitted to the intensive care unit had routine serial ultrasonographic assessment of the heart by one author (MM). Assessments of the heart were made using a Hewlett‐Packard SONOS 2000 with a 5.5/7.5 MHz transducer. The 7.5 MHz transducer was used for two dimensional studies, and the 5.5 MHz transducer was used for colour Doppler flow recordings. These examinations were started three hours after birth, with subsequent measurements at 12, 24, 36, 48, 72, and 96 hours of life, and on days 5, 6, 7, 14, 21, and 28. On initial physical and echocardiographic examination, none of the 52 infants was found to have any congenital abnormalities. Each echocardiographic estimate was expressed as the mean value of three to five measurements. Left ventricular function was assessed by M mode echocardiograms taken from the parasternal long axis view by the method of Sahn et al.13 Doppler measurements of LVO were by the method of Alverson et al.14 Left ventricular stroke volume was derived by dividing LVO by the heart rate measured on electrocardiograms that were recorded simultaneously.
Doppler assessment of pulmonary artery pressure (PAP) was performed using the corrected ratio of the pulmonary artery acceleration time to the right ventricular ejection time (AT/RVET(c)).15 A two dimensional image of the main pulmonary artery was visualised via the parasternal short axis view. The pulsed Doppler sample volume was then placed distal to the pulmonary valve, and the systolic Doppler waveform was recorded from the centre of the artery. Acceleration time was measured as the time interval between the waveform leaving the baseline and reaching its peak velocity. Right ventricular ejection time was the time interval between the waveform leaving and returning to the baseline. The AT/RVET(c) was calculated by dividing AT/RVET by the square root of the R‐R interval from a simultaneous electrocardiogram tracing. Pulsed Doppler measurement of the peak systolic velocity, end diastolic velocity, and time averaged velocity of the superior mesenteric artery (SMA) was performed as described by Deeg et al.16 Patent ductus arteriosus was diagnosed from clinical symptoms and serial echocardiography.
Statistical analysis was performed using the computer statistics package SPSS 6.1J for Macintosh (SPSS Japan, Tokyo, Japan). The Mann‐Whitney U test was used for continuous variables, and Fisher's exact test for discrete variables. Longitudinal comparisons of continuous variables were made by two way repeated measures analysis of variance and post hoc analysis. Differences with p<0.05 were considered significant.
Results
Tables 1 and 2 show the clinical features of the two groups. There were no significant differences in clinical profiles between the two groups. Rates of both clinical and histological maternal chorioamnionitis were significantly higher in the infectious group than in the control group. Mothers of 12 infants in the infectious group showed clinical or histological maternal chorioamnionitis; the remaining mother gave birth at home. Comparisons of laboratory findings showed that both mean whole white blood cell counts and serum C reactive protein concentrations were significantly higher in the infectious group than in the control group. Home oxygen therapy was required after hospital discharge for two infants in the infectious group.
Table 1 Comparison of baseline characteristics between the two groups.
| Infectious group (n = 13) | Control group (n = 39) | p Value | |
|---|---|---|---|
| Male/female | 7/6 | 18/21 | 0.75 |
| Gestational age (weeks) | 29.7 (2.2) | 29.6 (2.0) | 0.86 |
| Birth weight (g) | 1232 (194) | 1221 (191) | 0.87 |
| 1 minute Apgar | 7.2 (1.7) | 7.1 (1.7) | 0.80 |
| 5 minute Apgar | 8.5 (1.0) | 8.5 (0.8) | 0.79 |
| Outborn patients | 3 (23%) | 3 (8%) | 0.32 |
| Caesarean section | 11 (85%) | 37 (95%) | 0.55 |
| Antenatal steroids | 4 (31%) | 20 (51%) | 0.34 |
| Clinical chorioamnionitis | 8 (61%) | 9 (23%) | 0.02* |
| Histological chorioamnionitis | 10 (77%) | 6 (15%) | <0.0001*** |
Values are mean (SD) or number (%).
*p<0.05, ***p<0.001.
Table 2 Comparison of subsequent courses between the two groups.
| Infectious group (n = 13) | Control group (n = 39) | p Value | |
|---|---|---|---|
| Mechanical ventilation | 6 (46%) | 24 (61%) | 0.35 |
| Length of ventilation† (hours) | 31 (49) | 82 (178) | 0.11 |
| Length of oxygen treatment‡ (days) | 45 (34) | 34 (21) | 0.37 |
| Surfactant | 6 (46%) | 24 (61%) | 0.35 |
| Home oxygen therapy | 2 (15%) | 0 (0) | 0.10 |
| Laboratory findings | |||
| Arterial pH | 7.28 (0.10) | 7.31 (0.09) | 0.61 |
| Arterial base deficit (mmol/l) | 4.1 (3.6) | 4.1 (2.7) | 0.86 |
| First CK (IU/l) | 160 (108) | 195 (145) | 0.51 |
| First WBC (×109/l) | 24.5 (13.1) | 10.7 (6.1) | <0.0001*** |
| First haemoglobin (g/dl) | 14.9 (1.4) | 15.5 (2.4) | 0.43 |
| First IgM (mg/dl) | 77.5 (51.2) | 3.9 (4.3) | <0.0001*** |
| First CRP (mg/dl) | 0.28 (0.51) | 0.02 (0.04) | 0.002** |
Values are mean (SD) or number (%).
**p<0.01, ***p<0.001.
†Mean length of time only in infants who were successfully extubated.
‡Mean length of day only in infants who were discharged without oxygen.
CK, Creatine kinase; WBC, white blood cell count; CRP, C reactive protein.
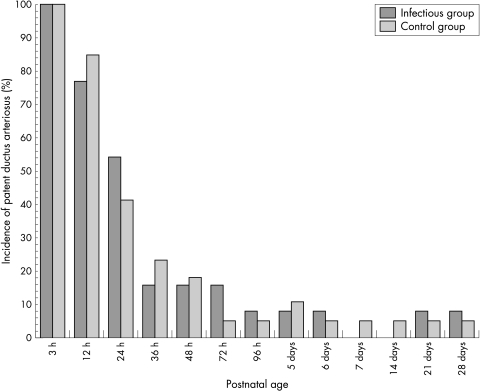
The incidence of CLD was significantly higher in the infectious group than in the control group (table 3). All four infants with CLD in the infectious group showed radiological findings on chest radiographs characterised by diffuse small cystic translucencies, which indicated congenital infections. The occurrence of septicaemia seemed to be higher in the infectious group, but the difference was not statistically significant. Figure 1 shows longitudinal comparisons of echocardiographically detected patent ductus arteriosus. The rate of patent ductus arteriosus by echocardiography was the same at all points of assessment between the two groups.
Table 3 Comparison of adverse clinical events between the two groups.
| Infectious group (n = 13) | Control group (n = 39) | p Value | |
|---|---|---|---|
| Pulmonary haemorrhage | 1 (8) | 2 (5) | >0.99 |
| Pneumothorax | 0 (0) | 2 (5) | >0.99 |
| Intraventricular haemorrhage | 1 (8) | 4 (10) | >0.99 |
| Periventricular leucomalacia | 1 (8) | 1 (3) | >0.99 |
| Septicaemia | 5 (38) | 7 (18) | 0.15 |
| Retinopathy of prematurity requiring photocoagulation | 2 (15) | 3 (8) | 0.79 |
| Chronic lung disease | 4 (30.8) | 1 (2.6) | 0.01** |
Values are number (%).
**p<0.01.
Figure 1 Longitudinal comparisons of echocardiographically detectable patent ductus arteriosus between the two groups in the early neonatal period.
Serial echocardiographic assessment revealed no difference in the time course of dimension of left ventricle, left ventricular end diastolic volume, left ventricular end systolic volume, left ventricular ejection fraction, left and right ventricular systolic time intervals, and heart rate between the two groups (data not shown).
Mean LVO and left ventricular stroke volume were both significantly higher in the infectious group than in the control group in the first 28 days of life (tables 4 and 5). There were no differences in the course of arterial blood pressure between the two groups (data not shown).
Table 4 Longitudinal comparisons of left ventricular output between the two groups.
| Postnatal age | Infectious group (n = 13) | Control group (n = 39) | p Value |
|---|---|---|---|
| 3 h | 222.5 (55.6) | 231.6 (70.6) | 0.73 |
| 12 h | 187.8 (50.9) | 153.7 (56.5) | 0.02* |
| 24 h | 197.2 (52.4) | 164.3 (57.9) | 0.06 |
| 36 h | 192.3 (43.6) | 177.1 (50.8) | 0.21 |
| 48 h | 178.2 (47.1) | 178.5 (46.7) | 0.86 |
| 72 h | 216.1 (69.3) | 173.2 (57.9) | 0.03* |
| 96 h | 184.4 (94.5) | 178.8 (60.7) | 0.68 |
| 5 days | 205.5 (50.9) | 179.6 (53.7) | 0.07 |
| 6 days | 203.9 (48.6) | 181.7 (43.8) | 0.17 |
| 7 days | 189.5 (52.5) | 171.4 (43.6) | 0.30 |
| 14 days | 191.9 (62.0) | 168.2 (49.8) | 0.24 |
| 21 days | 199.8 (58.7) | 171.2 (45.1) | 0.13 |
| 28 days | 182.0 (58.6) | 169.9 (51.1) | 0.62 |
Values are mean (SD) expressed as ml/kg/min.
*p<0.05.
Table 5 Longitudinal comparisons of left ventricular stroke volume between the two groups.
| Postnatal age | Infectious group (n = 13) | Control group (n = 39) | p Value |
|---|---|---|---|
| 3 h | 1.50 (0.47) | 1.47 (0.46) | 0.95 |
| 12 h | 1.37 (0.39) | 1.10 (0.43) | 0.02* |
| 24 h | 1.50 (0.45) | 1.18 (0.45) | 0.03* |
| 36 h | 1.41 (0.33) | 1.26 (0.37) | 0.17 |
| 48 h | 1.28 (0.30) | 1.24 (0.36) | 0.71 |
| 72 h | 1.54 (0.44) | 1.20 (0.38) | 0.02* |
| 96 h | 1.31 (0.65) | 1.26 (0.44) | 0.95 |
| 5 days | 1.50 (0.39) | 1.31 (0.39) | 0.08 |
| 6 days | 1.51 (0.34) | 1.31 (0.35) | 0.07 |
| 7 days | 1.41 (0.33) | 1.27 (0.35) | 0.13 |
| 14 days | 1.29 (0.35) | 1.19 (0.40) | 0.30 |
| 21 days | 1.37 (0.41) | 1.14 (0.31) | 0.06 |
| 28 days | 1.28 (0.57) | 1.13 (0.32) | 0.68 |
Values are mean (SD) expressed as ml/kg.
*p<0.05.
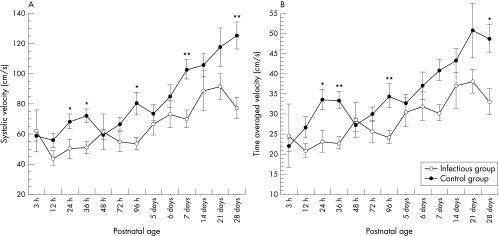
Table 6 shows the longitudinal comparison of AT/RVET(c) between the two groups. Mean values of AT/RVET(c) were significantly lower in the infectious group than in the control group. Figure 2 shows comparisons of the time course of SMA flow velocities. Mean systolic velocity and time averaged velocity were both significantly lower in the infectious group than in the control group within 28 days of birth, but there were no significant differences in end diastolic velocity and resistance index between the two groups.
Table 6 Longitudinal comparisons of corrected ratio of the pulmonary artery acceleration time to the right ventricular ejection time between the two groups.
| Postnatal age | Infectious group (n = 13) | Control group (n = 39) | p Value |
|---|---|---|---|
| 3 h | 0.44 (0.12) | 0.40 (0.11) | 0.31 |
| 12 h | 0.38 (0.09) | 0.41 (0.10) | 0.51 |
| 24 h | 0.40 (0.07) | 0.43 (0.09) | 0.27 |
| 36 h | 0.38 (0.08) | 0.44 (0.08) | 0.10 |
| 48 h | 0.38 (0.06) | 0.46 (0.10) | 0.01* |
| 72 h | 0.39 (0.06) | 0.43 (0.09) | 0.21 |
| 96 h | 0.39 (0.08) | 0.45 (0.08) | 0.04* |
| 5 days | 0.40 (0.10) | 0.43 (0.07) | 0.50 |
| 6 days | 0.40 (0.12) | 0.43 (0.10) | 0.14 |
| 7 days | 0.42 (0.10) | 0.42 (0.09) | 0.97 |
| 14 days | 0.41 (0.08) | 0.48 (0.10) | 0.04* |
| 21 days | 0.44 (0.07) | 0.49 (0.09) | 0.12 |
| 28 days | 0.42 (0.12) | 0.48 (0.09) | 0.04* |
Values are mean (SD).
*p<0.05.
Figure 2 Longitudinal comparisons of mean (SE) values of peak systolic velocity (A) and time averaged velocity (B) in the superior mesenteric artery between the two groups in the early neonatal period. *p<0.05, **p<0.01.
Discussion
This study shows that LVO and left ventricular stroke volume in infants suspected of having congenital infections because of raised concentrations of serum IgM at birth remained high throughout the early neonatal period. Although the true cause remains unclear, a significant increase in both white blood cell count and C reactive protein in infants in the infectious group suggests that they had already suffered from systemic inflammation at birth. Many investigators have reported that intrauterine amniotic fluid infection results in increased release of various inflammatory cytokines.11,12,13,14 Some of these cytokines may induce a significant increase in cardiac output directly or by stimulating other mediators such as cortisol or prostaglandins.3,4 From the results of our study, we suggest that the high LVO in VLBW infants with intrauterine infection in the early neonatal period may result from a continuation of the fetal systemic inflammatory response.
The type of prenatal infection that leads to acute respiratory disease in premature infants is still controversial,1,4,5,17,18 but it is now widely established that intrauterine infection is a major risk factor for chronic lung instability in later life.3,5,6 Fujimura et al7,8 reported that many preterm infants with maternal chorioamnionitis who showed raised IgM concentrations at birth developed Wilson‐Mikity syndrome, which is known to be a unique and intractable form of CLD. The incidence of CLD in infants in the infectious group in the present study was significantly higher than in the control, and all the infants with CLD may have similar clinical and radiological findings to those described by Fujimura et al.7,8 Oxygen and high airway pressure due to long term mechanical ventilation is known to injure and alter the pulmonary vasculature in premature infants with CLD.19,20 It is generally established that these acquired pulmonary vascular lesions may lead to an increase in pulmonary vascular resistance through narrowing of the vessel diameter and decreased vascular compliance, and the development of pulmonary hypertension in infants with CLD.19,20
Direct assessment of PAP by cardiac catheterisation is difficult in premature infants, but Doppler echocardiography has provided doctors with a non‐invasive method by which PAP can be measured. AT/RVET, which can be estimated non‐invasively from Doppler pulmonary artery waveforms, has been shown to correlate negatively with PAP in adults,21 children, older infants, and, recently, premature infants.15,23,24,25,26 Some investigators have encountered several methodological problems in the clinical assessment of AT/RVET. Skinner et al22 suggested that AT/RVET values in newborn infants with persistent pulmonary hypertension bear little relation to PAP assessed by other methods and may be of little value because of poor reproducibility. In our previous studies,23 it was also reported that AT/RVET in VLBW infants depended significantly on gestational age, and did not correlate well with PAP assessed by tricuspid regurgitation in the early neonatal period.
However, the importance of AT/RVET in the management of premature infants with CLD is supported by many authors.24,25,26 Several studies have shown that early pulmonary hypertension in premature infants, as assessed by AT/RVET, is a good predictor of late onset CLD.26,27 In this report, it was found that AT/RVET(c) values were lower in infants with prenatal infections than in controls during the first month of life, and these changes may resemble the previously reported course of AT/RVET in infants with CLD.26,27 Although it remains to be investigated whether AT/RVET(c) actually reflects PAP in premature infants, we suggest that the low AT/RVET(c) values in the first 28 days of life in VLBW infants with prenatal infections represent latency of pulmonary hypertension. In the infectious group, four of 13 infants went on to develop CLD, but the remaining nine had no dependency on oxygen in the later neonatal period. If AT/RVET(c) correlated inversely with PAP in premature infants, the infants with prenatal infections may have pulmonary hypertension for several weeks after birth irrespective of their dependency on oxygen, which could only be detected by assessment of AT/RVET(c) using serial Doppler echocardiography.
What is already known on this topic
There are no reports on LVO and AT/RVET in infants with intrauterine infections
Some studies have shown that flow velocity in the splanchnic artery may be increased in preterm neonates with perinatal sepsis
What this study adds
Early LVO values were significantly higher and mean AT/RVET(c) values were significantly lower in infants with intrauterine infection than in controls
Both peak systolic velocity and time averaged velocity in the superior mesenteric artery were significantly decreased in infants with intrauterine infection compared with controls
In this study, a significant decrease in SMA flow velocity was shown in infants with suspected prenatal infection within 28 days of birth.16,27,28,29 Several authors have described how gastrointestinal blood flow velocity in full term and preterm neonates, as assessed by ultrasound, is influenced by asphyxia,27 intrauterine growth retardation, congenital heart disease, necrotising enterocolitis,16,28 and cystic periventricular leucomalacia. Kempley et al29 showed that, in preterm neonates with perinatal sepsis, the flow velocity in the coeliac artery increased while the pulsatility index of both the coeliac artery and SMA decreased, suggesting the possibility of splanchnic haemodynamic disturbance caused by inflammation resulting from the early infectious event. In the present analysis, the flow velocity of SMA decreased in infants with prenatal infections, and the resistance indices of SMA seemed to be similar to those in controls. These findings may appear to be inconsistent with the report of Kempley et al.29 Their assessment of Doppler flow velocities, however, was made at only a single point within 24 hours of birth and differed significantly from our longitudinal assessment from birth to 28 days. Akinbi et al27 reported a significant reduction in SMA flow velocities in proportion to the severity of the infant's asphyxia. It is likely that the significant decrease in SMA flow velocity in our infants with suspected prenatal infection was associated with intrauterine fetal exposure to ischaemia and hypoxia caused by the fetal inflammatory response.
In summary, LVO in infants suspected of having intrauterine infection because of raised serum IgM at birth was significantly higher in the early neonatal period, whereas both AT/RVET values and SMA flow velocities in the infectious infants were significantly decreased compared with controls. We suggest that this unique status of the systemic/pulmonary circulation, namely high output, high PAP, and low organ flow, may be caused by a continuation of the increase in cytokine release derived from the fetal inflammatory response. These findings in premature infants with suspected intrauterine infection may be of value in the management of early cardiopulmonary circulation.
Abbreviations
AT/RVET(c) - corrected ratio of the pulmonary artery acceleration time to the right ventricular ejection time
CLD - chronic lung disease
LVO - left ventricular output
PAP - pulmonary artery pressure
SMA - superior mesenteric artery
VLBW - very low birthweight
Footnotes
Competing interests: none declared
References
- 1.Romero R, Gomez R, Ghezzi F.et al A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998179186–193. [DOI] [PubMed] [Google Scholar]
- 2.Gomez R, Romero R, Ghezzi F.et al The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998179194–202. [DOI] [PubMed] [Google Scholar]
- 3.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate 20038385–96. [DOI] [PubMed] [Google Scholar]
- 4.Hitti J, Krohn M A, Patton D L.et al Amniotic fluid tumor necrosis factor and the risk of respiratory distress syndrome among preterm infants. Am J Obstet Gynecol 199717750–56. [DOI] [PubMed] [Google Scholar]
- 5.Watterberg K L, Demers L M, Scott S M.et al Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 199697210–215. [PubMed] [Google Scholar]
- 6.Lyon A. Chronic lung disease of prematurity. The role of intra‐uterine infection. Eur J Pediatr 2000159798–802. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura M, Takeuchi T, Ando M.et al Elevated immunoglobulin M levels in low birth‐weight neonates with chronic respiratory insufficiency. Early Hum Dev 1983927–32. [DOI] [PubMed] [Google Scholar]
- 8.Fujimura M, Takeuchi T, Kitajima H.et al Chorioamnionitis and serum IgM in Wilson‐Mikity syndrome. Arch Dis Child 1989641379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill A B, Weindling A M. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child 19936817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans N, Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 199674F88–F94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner J R, Boys R J, Hunter S.et al Pulmonary and systemic arterial pressure in hyaline membrane disease. Arch Dis Child 199267366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams C B, Cole F S. Immunology of the fetus and newborn. In: Taeusch HW, Ballard RA, Gleason CA, eds. Avery's diseases of the newborn. 8th ed. Philadelphia: Elsevier Saunders, 2005447–479.
- 13.Sahn D J, DeMaria A, Kisslo J.et al Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978581072–1083. [DOI] [PubMed] [Google Scholar]
- 14.Alverson D C, Eldridge M, Dillion T.et al Noninvasive pulsed Doppler determination of cardiac output in neonates and children. J Pediatr 198210146–50. [DOI] [PubMed] [Google Scholar]
- 15.Hamdan A H, Shaw N J. Changes in pulmonary artery pressure in infants with respiratory distress syndrome following treatment with Exosurf. Arch Dis Child Fetal Neonatal Ed 199572F176–F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeg K H, Rupprecht T, Schmid E. Doppler sonographic detection of increased flow velocities in the celiac trunk and superior mesenteric artery in infants with necrotizing enterocolitis. Pediatr Radiol 199323578–582. [DOI] [PubMed] [Google Scholar]
- 17.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin‐1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997992992–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watterberg K L, Scott S M, Naeye R L. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics 199799e6. [DOI] [PubMed] [Google Scholar]
- 19.Jones R, Zapol W M, Reid L. Oxygen toxicity and restructuring of pulmonary arteries: a morphometric study. Am J Pathol 1985121212–223. [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman G, Perkin R M, Anas N G.et al Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr 198811267–72. [DOI] [PubMed] [Google Scholar]
- 21.Kitabatake A, Inoue M, Asao M.et al Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 198368302–309. [DOI] [PubMed] [Google Scholar]
- 22.Skinner J R, Hunter S, Hey E N. Haemodynamic features at presentation in persistent pulmonary hypertension of the newborn and outcome. Arch Dis Child Fetal Neonatal Ed 199674F26–F32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murase M, Ishida A. Serial pulsed Doppler assessment of pulmonary artery pressure in very low birth‐weight infants. Pediatr Cardiol 200021452–457. [DOI] [PubMed] [Google Scholar]
- 24.Gill A B, Weindling A M. Pulmonary artery pressure changes in the very low birthweight infant developing chronic lung disease. Arch Dis Child 199368303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subhedar N V, Hamdan A H, Ryan S W.et al Pulmonary artery pressure: early predictor of chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed 199878F20–F24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subhedar N V, Shaw N J. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 200082F243–F247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akinbi H, Abbasi S, Hilpert P L.et al Gastrointestinal and renal blood flow velocity profile in neonates with birth asphyxia. J Pediatr 1994125625–627. [DOI] [PubMed] [Google Scholar]
- 28.Kempley S T, Gamsu H R. Superior mesenteric artery blood flow velocity in necrotizing enterocolitis. Arch Dis Child 199267793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempley S T, Murdoch E. Splanchnic haemodynamic disturbances in perinatal sepsis. Arch Dis Child Fetal Neonatal Ed 200083F139–F142. [DOI] [PMC free article] [PubMed] [Google Scholar]