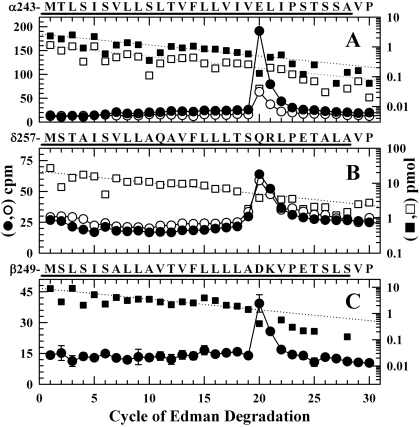
Fig. 7.
Photolabeling after rapid-freezing of nAChRs equilibrated with [3H]azietomidate and agonist. 3H (•, ○) and PTH-amino acids (▪, □) released during sequence analysis of nAChR subunit fragments beginning at the amino terminus of αM2 (A), δM2 (B), and βM2 (C). The primary amino acid sequences are shown above each panel. nAChR-rich membranes (12 mg/condition), pre-equilibrated with 18 μM [3H]azietomidate either without (filled symbols) or with (open symbols) 10 mM Carb, were exposed to 10 mM Carb for 50 ms and then rapidly frozen for photolabeling. As described under Materials and Methods, the fragments containing αM2 were isolated by reversed-phase HPLC from EndoLys-C digests of αV8-20; the fragments containing at δM2 or βM2 were isolated from an EndoLys-C digests (δ subunit) or trypsin digests (β subunit) by Tricine-gel SDS-PAGE followed by reversed-phase HPLC. A, the primary sequence began at αMet-243 (▪, I0 = 2.7 pmol; □, I0 = 1.2 pmol), and the peak of 3H release in cycle 20 indicated labeling of αGlu-262. B, the primary sequence began at δMet-257 [□, I0 = 22 pmol; not pre-equilibrated with Carb, I0 = 19 pmol (not shown)]. The 3H release in cycle 20 indicated labeling at δGln-276. C, the primary sequence began at the N terminus of βM2 (▪, I0 = 9.2 pmol) and a secondary sequence began at βLys-216 at the N terminus of βM1 (I0 = 5.1 pmol, not shown). The 3H release in cycle 20 indicated labeling at βAsp-268.