Abstract
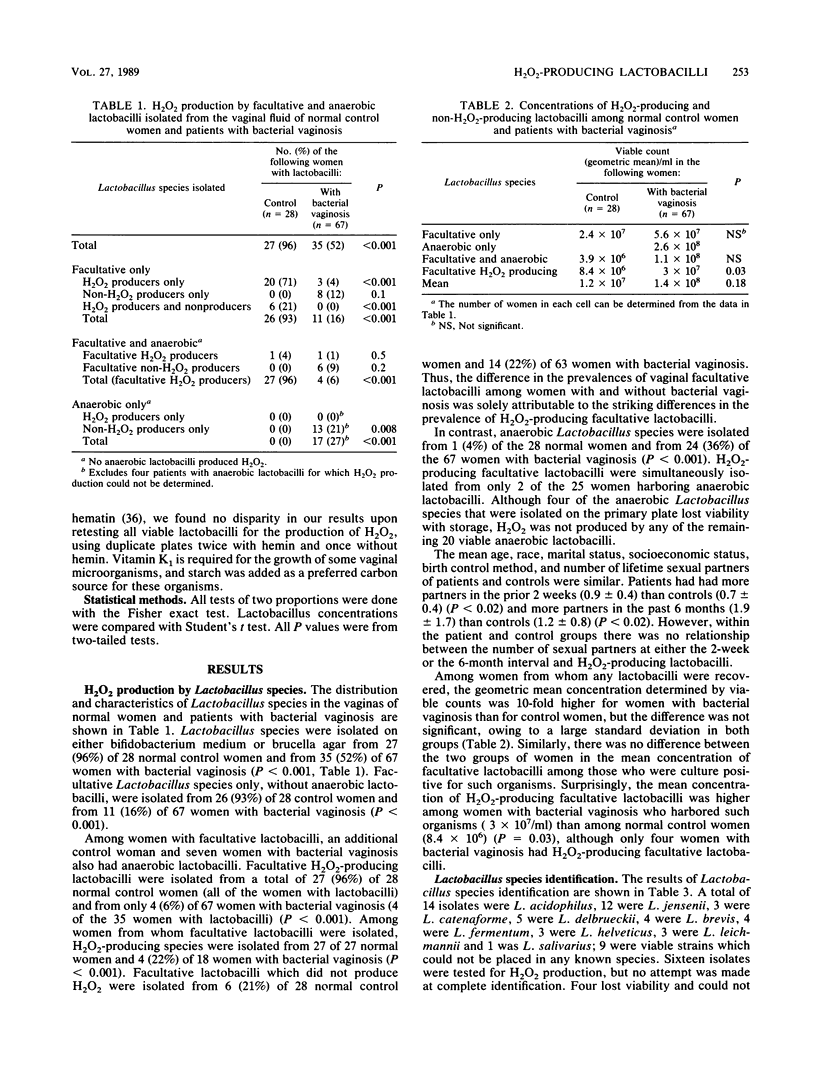
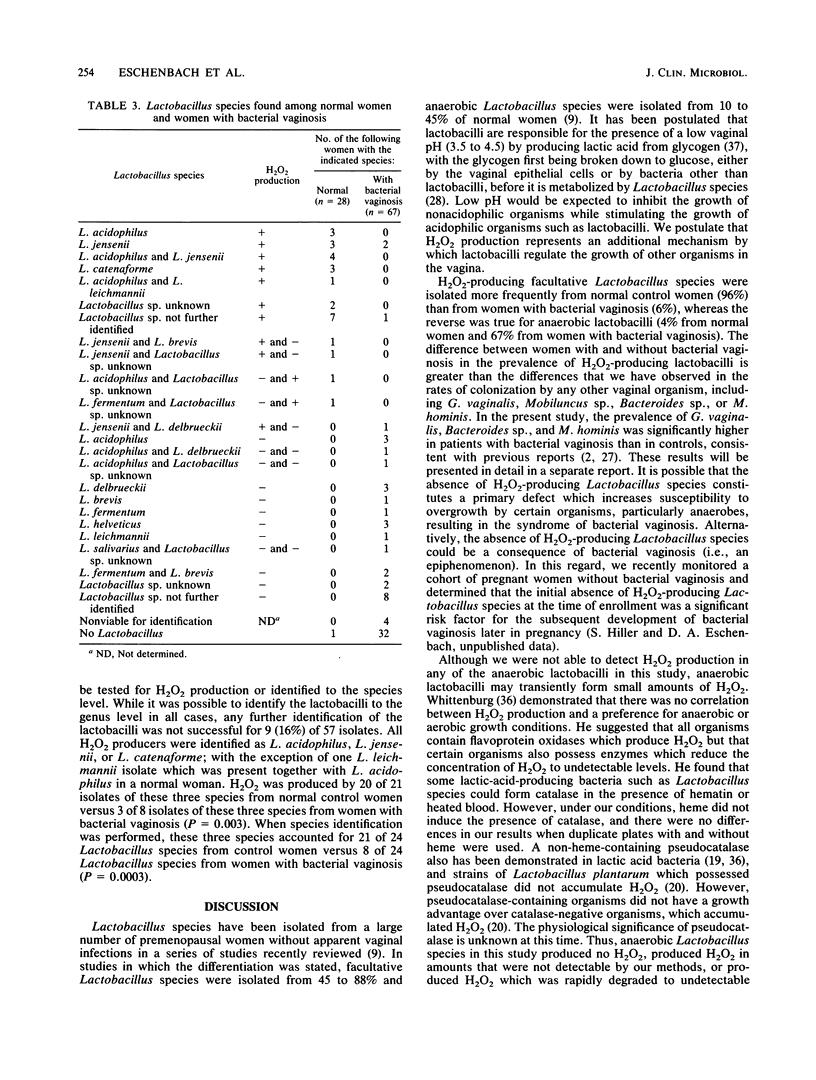
A predominance of Lactobacillus species in the vaginal flora is considered normal. In women with bacterial vaginosis, the prevalence and concentrations of intravaginal Gardnerella vaginalis and anaerobes are increased, whereas the prevalence of intravaginal Lactobacillus species is decreased. Because some lactobacilli are known to produce hydrogen peroxide (H2O2), which can be toxic to organisms that produce little or no H2O2-scavenging enzymes (e.g., catalase), we postulated that an absence of H2O2-producing Lactobacillus species could allow an overgrowth of catalase-negative organisms, such as those found among women with bacterial vaginosis. In this study, H2O2-producing facultative Lactobacillus species were found in the vaginas of 27 (96%) of 28 normal women and 4 (6%) of 67 women with bacterial vaginosis (P less than 0.001). Anaerobic Lactobacillus species (which do not produce hydrogen peroxide) were isolated from 24 (36%) of 67 women with bacterial vaginosis and 1 (4%) of 28 normal women (P less than 0.001). The production of H2O2 by Lactobacillus species may represent a nonspecific antimicrobial defense mechanism of the normal vaginal ecosystem.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsel R., Totten P. A., Spiegel C. A., Chen K. C., Eschenbach D., Holmes K. K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983 Jan;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Blackwell A. L., Fox A. R., Phillips I., Barlow D. Anaerobic vaginosis (non-specific vaginitis): clinical, microbiological, and therapeutic findings. Lancet. 1983 Dec 17;2(8364):1379–1382. doi: 10.1016/s0140-6736(83)90920-0. [DOI] [PubMed] [Google Scholar]
- Blaine J. A., Heald P. J., Mack A. E., Shaw C. E. Peroxidase in human cervical mucus during the menstrual cycle. Contraception. 1975 Jun;11(6):677–680. doi: 10.1016/0010-7824(75)90064-5. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Dahiya R. S., Speck M. L. Hydrogen peroxide formation by lactobacilli and its effect on Staphylococcus aureus. J Dairy Sci. 1968 Oct;51(10):1568–1572. doi: 10.3168/jds.S0022-0302(68)87232-7. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- GARDNER H. L., DUKES C. D. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 1955 May;69(5):962–976. [PubMed] [Google Scholar]
- Hill G. B., Eschenbach D. A., Holmes K. K. Bacteriology of the vagina. Scand J Urol Nephrol Suppl. 1984;86:23–39. [PubMed] [Google Scholar]
- Holinka C. F., Gurpide E. Peroxidase activity in glands and stroma of human endometrium. Am J Obstet Gynecol. 1980 Nov 15;138(6):599–603. doi: 10.1016/0002-9378(80)90073-3. [DOI] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. Catalase of the Lacto-bacillaceae. J Bacteriol. 1962 Apr;83:936–938. doi: 10.1128/jb.83.4.936-938.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E. C., Henderson W. R., Klebanoff S. J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980 Mar;124(3):1378–1382. [PubMed] [Google Scholar]
- Klebanoff S. J., Belding M. E. Virucidal activity of H2O2-generating bacteria: requirement for peroxidase and a halide. J Infect Dis. 1974 Mar;129(3):345–348. doi: 10.1093/infdis/129.3.345. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Smith D. C. Peroxidase-mediated antimicrobial activity of rat uterine fluid. Gynecol Invest. 1970;1(1):21–30. doi: 10.1159/000301903. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Smith D. C. The source of H2O2 for the uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):236–242. doi: 10.1093/biolreprod/3.2.236. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983 May 25;258(10):6015–6019. [PubMed] [Google Scholar]
- Liem H. H., Cardenas F., Tavassoli M., Poh-Fitzpatrick M. B., Muller-Eberhard U. Quantitative determination of hemoglobin and cytochemical staining for peroxidase using 3,3',5,5'-tetramethylbenzidine dihydrochloride, a safe substitute for benzidine. Anal Biochem. 1979 Oct 1;98(2):388–393. doi: 10.1016/0003-2697(79)90157-x. [DOI] [PubMed] [Google Scholar]
- Mackenzie D. W. Serum tube identification of Candida albicans. J Clin Pathol. 1962 Nov;15(6):563–565. doi: 10.1136/jcp.15.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W. M., Hayes C. H., Rosner B., Evrard J. R., Crockett V. A., Alpert S., Zinner S. H. Vaginal colonization with Corynebacterium vaginale (Haemophilus vaginalis). J Infect Dis. 1977 Dec;136(6):740–745. doi: 10.1093/infdis/136.6.740. [DOI] [PubMed] [Google Scholar]
- Reite B., Oram J. D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967 Oct 28;216(5113):328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- STEWART-TULL D. E. EVIDENCE THAT VAGINAL LACTOBACILLI DO NOT FERMENT GLYCOGEN. Am J Obstet Gynecol. 1964 Mar 1;88:676–679. doi: 10.1016/0002-9378(64)90898-1. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Combs R. S. Enhancement of Ureaplasma urealyticum growth on a differential agar medium (A7B) by a polyamine, putrescine. J Clin Microbiol. 1979 Dec;10(6):931–933. doi: 10.1128/jcm.10.6.931-933.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler J. S., Childs R. E., Bardsley W. G. Peroxidase from human cervical mucus. The isolation and characterisation. Eur J Biochem. 1976 Jun 1;65(2):325–331. doi: 10.1111/j.1432-1033.1976.tb10345.x. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Amsel R., Eschenbach D., Schoenknecht F., Holmes K. K. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980 Sep 11;303(11):601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- THOMPSON R., JOHNSON A. The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci. J Infect Dis. 1951 Jan-Feb;88(1):81–85. doi: 10.1093/infdis/88.1.81. [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Thomason J. L., Kunigk A., Khan-Dawood F. S., Kirschner C. V., Spellacy W. N. Guaiacol peroxidase levels in human cervical mucus: a possible predictor of ovulation. Contraception. 1982 Jan;25(1):59–67. doi: 10.1016/0010-7824(82)90019-1. [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Trujillo Y. P., Fernandez B. B., Bardawil W. A., Kunigk A., Spellacy W. N. Distribution of guaiacol peroxidase in human endometrium and endocervical epithelium during the menstrual cycle. J Clin Endocrinol Metab. 1982 May;54(5):991–997. doi: 10.1210/jcem-54-5-991. [DOI] [PubMed] [Google Scholar]
- VON KAULLA K. N., AIKAWA J. K., BRUNS P. D., WIKLE W. T. Secretory function of the human uterine cervix; studies with radioisotopes. Fertil Steril. 1957 Sep-Oct;8(5):444–454. doi: 10.1016/s0015-0282(16)32823-0. [DOI] [PubMed] [Google Scholar]
- Vontver L. A., Eschenbach D. A. The role of Gardnerella vaginalis in nonspecific vaginitis. Clin Obstet Gynecol. 1981 Jun;24(2):439–460. doi: 10.1097/00003081-198106000-00009. [DOI] [PubMed] [Google Scholar]
- WHEATER D. M., HIRSCH A., MATTICK A. T. R. Possible identity of lactobacillin with hydrogen peroxide produced by lactobacilli. Nature. 1952 Oct 11;170(4328):623–624. doi: 10.1038/170623a0. [DOI] [PubMed] [Google Scholar]
- WHITTENBURY R. HYDROGEN PEROXIDE FORMATION AND CATALASE ACTIVITY IN THE LACTIC ACID BACTERIA. J Gen Microbiol. 1964 Apr;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]
- Wylie J. G., Henderson A. Identity and glycogen-fermenting ability of lactobacilli isolated from the vagina of pregnant women. J Med Microbiol. 1969 Aug;2(3):363–366. doi: 10.1099/00222615-2-3-363. [DOI] [PubMed] [Google Scholar]


