Abstract
GnRH acts via GnRH receptors (GnRH-R) in the pituitary to cause the release of gonadotropins that regulate vertebrate reproduction. In the teleost fish, Haplochromis burtoni, reproduction is socially regulated through the hypothalamus-pituitary-gonadal axis, making the pituitary GnRH-R a likely site of action for this control. As a first step toward understanding the role of GnRH-R in the social control of reproduction, we cloned and sequenced candidate GnRH-R complementary DNAs from H. burtoni tissue. We isolated a complementary DNA that predicts a peptide encoding a G protein-coupled receptor that shows highest overall identity to other fish type I GnRH-R (goldfish IA and IB and African catfish). Functional testing of the expressed protein in vitro confirmed high affinity binding of multiple forms of GnRH. Localization of GnRH-R messenger RNA using RT-PCR revealed that it is widely distributed in the brain and retina as well as elsewhere in the body. Taken together, these data suggest that this H. burtoni GnRH receptor probably interacts in vivo with all three forms of GnRH.
In vertebrates, reproduction depends on many factors, including photoperiod, resource abundance, and conspecific presence. Ultimately, all of these factors exert their effects via a final common pathway, the hypothalamus-pituitary-gonadal axis. An essential step in this pathway is GnRH, a peptide hormone released from the hypothalamus into the pituitary where it causes the release of gonadotropins. As is well known, gonadotropins stimulate gonadal maturation and trigger the release of androgen hormones.
A pituitary GnRH receptor (GnRH-R) complementary DNA (cDNA) was first isolated in the mouse, and its functionality was confirmed via expression in Xenopus oocytes (1, 2). Subsequently, GnRH-Rs have been isolated in many mammalian species (3), and recently full-length clones have been obtained from nonmammalian species, including Xenopus laevis (4), African catfish (5), and two GnRH-R forms in the goldfish (6). These three fish GnRH receptors share approximately 70% amino acid identity and are about 42% identical to the human GnRH-R (6). The genomic DNA of several species contains duplicate forms of GnRH-R (7), but the question of whether multiple GnRH-Rs are typically expressed in diploid species is still unresolved (8).
Previous work from our laboratory has shown that reproductive state is socially regulated in Haplochromis burtoni. Dominant males have larger testes and more mature sperm than nondominant males (9). GnRH-Rs seem a likely site for social control of reproduction. As a first step in testing this hypothesis, we characterized a GnRH-R in H. burtoni.
Materials and Methods
Animals
Animals reared from wild-caught stock were maintained according to the animal care guidelines of Stanford University. For this work, only females and territorially dominant adult male fish were used.
RNA isolation, amplification, and analysis of GnRH-R
RT and cDNA amplification were performed as described previously (10) with the following differences. Total RNA was extracted from the brains of male and female H. burtoni. A nested PCR protocol was employed, using the following primers based on conserved regions of both goldfish GnRH-R subtypes (6) and the catfish GnRH-R (5): FH1 (5′-ATGACITT(C/T)(A/G)TIGTIATGCCI(T/C)T-3′) and FH5 (5′-GG(T/C)TG(A/G)AACCA(A/G)TACCA(G/T/A)ATICC-3′) for the primary reaction, and FH3 (5′-AA(C/T)GTIACIGTICA(A/G)TGGTA(C/T)GC-3′) and FH4 (5′-GCIA(G/A)IA(G/A)(A/G)TA(A/G)TAIGGIGTC-CA(A/G)C-3′) for the secondary reaction. Both reactions were performed in a Rapidcycler (Idaho Technologies, Idaho Falls, ID) with a 55 C-50 C touchdown protocol as follows: 15-sec denaturation at 94 C, followed by 35 cycles of 0-sec denaturation at 94 C, 0-sec annealing (55 C-50 C), and 15-sec extension at 72 C. These reactions yielded a single product, as revealed by gel electrophoresis and sequencing.
To obtain the complete cDNA, rapid amplification of 5′- and 3′-cDNA ends (5′- and 3′-RACE) was performed with hypothalamic total RNA using the SMART cDNA Synthesis Kit (CLONTECH Laboratories, Inc., Palo Alto, CA). The RACE products from this cDNA (Marathon kit, CLONTECH Laboratories, Inc.) were sequenced and found to contain the start and end codons. New primers, specific for the 5′- and 3′-untranslated regions of the H. burtoni GnRH-R, were then used to obtain the full-length cDNA.
The nucleotide sequence and predicted peptide sequence were compared with known GnRH-R cDNAs in GenBank (www.ncbi.nlm.nih.gov/BLAST). The sequences were aligned with ALIGN and CLUSTALW (http://dot.imgen.bcm.tmc.edu:9331). Transmembrane domains of the H. burtoni sequence were predicted by SOUSI (http://azusa.proteome.bio.tuat.ac.jp/sosui).
Cloning of GnRH-R cDNA
The full-length PCR product was purified and blunt end-cloned into pBluescript II (Stratagene, La Jolla, CA). After ligation, the recombinant plasmid was used to transform competent Escherichia coli XL1-Blue MRF’ cells (Stratagene). After overnight incubation on Luria Bertoni broth-ampicillin plates and blue-white color selection, six transformants were chosen for further analysis. The orientation of the inserts was confirmed by sequencing with T3 and T7 primers.
Transient transfection of COS-1 cells and functional assay
The H. burtoni GnRH-R cDNA was ligated into pcDNA/Amp vector (Invitrogen, San Diego, CA) and transfected into COS-1 cells as previously described (6). The functionality of the putative GnRH-R was tested by measuring inositol phosphate (IP) production in response to GnRH. The IP assay was performed as described previously (6). The GnRH agonists tested appear in Table 1. Among these are the mammalian releasing form of GnRH (mGnRH1) and the three forms of GnRH found in H. burtoni: sbGnRH1 (or seabream GnRH) in the hypothalamus, GnRH2 (or chicken-II GnRH) in the midbrain, and GnRH3 (or salmon GnRH) in the telencephalon (11). Data points were determined in duplicate, and the EC50 values represent the mean of three separate experiments.
TABLE 1.
EC50 values of GnRH agonists for inositol phosphate production in COS-1 cells transiently transfected with the H. burtoni GnRH-R
| Peptide | EC50 (nM) |
|---|---|
| [His5,Trp7,Tyr8]GnRH (GnRH2) | 0.31 ± 0.12 |
| [Trp7,Leu8]GnRH (GnRH3) | 238.8 ± 128.5 |
| [Arg8]GnRH (mGnRH1) | 600 ± 410.2 |
| [Ser8]GnRH (sbGnRH1) | 1696.6 ± 515.4 |
| [His8]GnRH | 167 ± 34.7 |
| [Tyr8]GnRH | 75.6 ± 18.9 |
| [His5,D-Arg6,Leu8]GnRH | 93.7 ± 75.8 |
| [D-Arg6,Trp7,Tyr8]GnRH | 0.15 ± 0.02 |
| [D-Arg6,Trp7,Leu8]GnRH | 23.3 ± 12.5 |
Data are calculated as the mean of three separate experiments.
GnRH binding assay
[His5,D-Tyr6]GnRH was radioiodinated as previously described (12). Transfected cells were washed twice for 5 min each time at 4 C in buffer I (140 mM NaCl, 4 mM KCl, 20 mM HEPES, 8.6 mM glucose, 1 mM CaCl2, 1 mM MgCl2, and 0.1% fatty acid-free BSA, pH 7.4) followed by incubation with 100,000 cpm 125I-labeled [His5,D-Tyr6]GnRH in 0.5 ml buffer I in the absence or presence of unlabeled ligand for 5 h at 4 C. Unbound label was removed by washing twice in buffer I, and cells were removed from the plates with 1 ml 0.5 M NaOH. Nonspecific binding was determined by measuring maximum binding on untransfected cells. Data points were determined in duplicate, and the IC50 values represent the mean of three separate experiments.
GnRH-R messenger RNA (mRNA) localization
PCR was performed on tissue from adult H. burtoni (gill, heart, intestine, kidney, liver, muscle, ovary, retina, spleen, and testes). In addition, brains from six adult fish were partitioned for RT-PCR analysis: cerebellum, pituitary, optic tectum, telencephalon, and the remaining brain, including the hypothalamus. cDNA was synthesized as described above, except 0.5 μg RNA was used from pituitary and 1.0 μg from cerebellum. Primers specific to the H. burtoni GnRH-R were designed, Hb5′ (5*-TGACAGTGCAGTGGTATGGTG-3*) and Hb3′ (5*-TCAGAGTCTTCATCCGAGCCTTTG-3*), which generated a 483-bp PCR product. These primers flank an intron in the GnRH-R sequence, so any amplicons from possible genomic contamination can be eliminated. DNA Positive controls were performed using primers for glycerol-3-phosphate dehydrogenase, the ubiquitous housekeeping gene. Every reaction was performed at least twice. In addition, negative controls were performed using RNA (without RT) as a template in our amplification protocol. None of these reactions produced products.
Phylogenetic analysis of GnRH-R
To situate the H. burtoni GnRH-R with respect to previously cloned GnRH receptors, phylogenetic analysis was used. The predicted sequences of GnRH-R polypeptides were aligned using ClustalW 1.8 (http://dot.imgen.bcm.tmc.edu:9331/multialign/multialign.html) and were subsequently converted to the NEXUS format with READSEQ (http://dot.imgen.bcm.tmc.edu:9331/seq-util/seq-util.html). The trees were then generated using PAUP* 4.0b3a (13). To test robustness, trees were generated using neighbor-joining, star decomposition, heuristic, and branch and bound methods, all of which produced very similar results. These analyses were performed both on sequences lacking the relatively unconserved extra- and intracellular tails and on full-length sequences, both of which yielded similar relationships in the trees. Full species names and GenBank accession numbers for the receptor cDNAs are as follows: African catfish, Clarias gariepinus, O42329; goldfish, Carassius auratus, IA: AAD20001 and IB: AAD20002; eel, Anguilla japonica, BAB11961; pig, Sus scrofa, P49922; cow, Bos taurus, AAC48857; mouse, Mus musculus, AAB59636; sheep, Ovis aries, AAC37336; rat, Rattus norvegicus, AAC27349; and human, Homo sapiens, NP 000397. Also, the sequences for possum (Trichosurus vulpecula) GnRH-R (14) and frog (Xenopus laevis) type I GnRH-R (4) were used. The following sequences were unpublished at the time of this writing: perciform fishes Seriola dumerilii (CAB65407) and striped bass (Morone saxatilis, AAF28464), a caecilian amphibian (Typhlonectes natans, AAD49750), horse (Equus caballus, O18821), and type II from Xenopus (Troskie, B., N. Illing, and R. Millar, unpublished data). A fragment (33 amino acids) of a putative type II GnRH-R gene from H. burtoni genomic DNA was included in the tree for the purpose of illustrating the evolutionary divergence of the two types (see Discussion). The sequence for this putative type II GnRH-R gene has been submitted to GenBank (Accession No. AF356598).
Results
GnRH-R structure
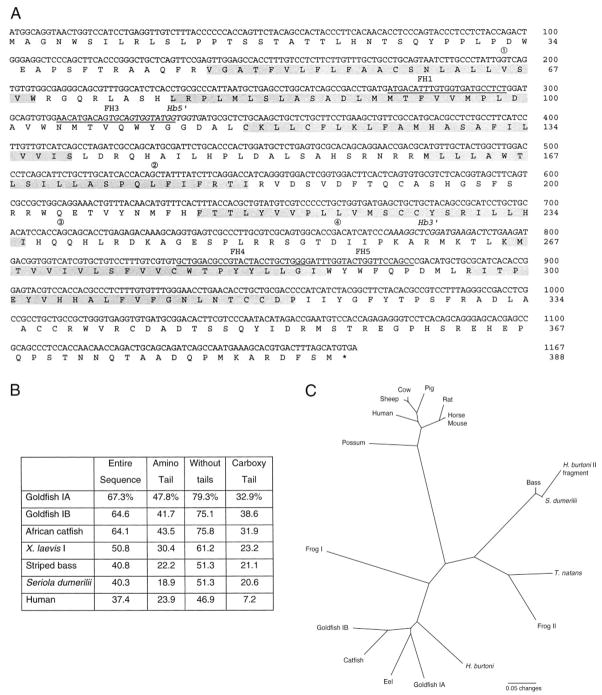
Analysis of the cDNA sequence obtained from H. burtoni strongly supports that it is a GnRH-R. Amplification, with primers based on fish type I sequences, yielded a single cDNA (GenBank Accession No. AY028476) whose sequence predicts a G protein-coupled receptor with seven transmembrane domains (Fig. 1A). This sequence shows highest homology to sequences from other GnRH receptors, followed by sequences from vasopressin and oxytocin receptors. In particular, the cDNA we found is most similar to the GnRH-R sequence reported in goldfish and African catfish (Fig. 1B). Similar to the other teleost GnRH-R receptors, but in contrast to mammalian GnRH-R forms, the H. burtoni GnRH-R includes approximately 50 intracellular amino acid residues at the C-terminus. The intracellular tail, which is lacking in mammals, is never highly conserved, and the extracellular tail shows at most a modest conservation.
Fig. 1.
A, GnRH-R nucleotide and predicted peptide sequences from H. burtoni. Transmembrane domains are shaded. Variant transcripts were found missing the bases between 1 and 2 and between 3 and 4 (positioned above the sequence here). Priming sites are indicated for the nested homology-based primers (underlined) and the H. burtoni-specific primers (italics). B, Comparison of predicted amino acid sequence homology among H. burtoni, several other teleost species, an amphibian (X. laevis), and a mammal, shown as percent identity. Sequence comparisons were made for the whole sequence, the amino extracellular tail alone, the whole sequence without the tails, and the carboxyl intracellular tail alone. C, Phylogenetic comparison of available cDNA sequences for GnRH-R. Note that nonmammalian sequences are separated into type I and type II (7). The H. burtoni GnRH-R reported here is type I, although an uncharacterized genomic DNA fragment (included here for comparison) aligns with type II sequences.
Although the gene structure of this H. burtoni GnRH-R was not studied directly, the locations of four introns can be inferred from two variant transcripts we found (Fig. 1A), as has been done for the mouse GnRH-R sequence (15). A cloned variant was missing the sequence from base 196 (TATTG/intron/GTCAG) to base 528 (CACAG/intron/CTATT). The latter position corresponds to an intron found in GnRH-R genes in humans, rats, mice, sheep, and even a GnRH-R-like gene from Drosophila melanogaster (15–19). This putative conserved intron corroborates phylogenetic analysis (see below) regarding GnRH-R in H. burtoni. Another variant, amplified from retinal cDNA in addition to the wild-type GnRH-R, was missing the sequence from base 710 (CCACC/intron/AGCAG) to base 762 (GCACC/intron/GACAT).
Phylogenetic relationships
The comparison of GnRH-R sequences between H. burtoni and other teleost fish species (Fig. 1B) suggests that there may be two distinct types. Specifically, the H. burtoni cDNA sequence shows higher homology with those of goldfish and African catfish than to those of S. dumerilii and striped bass, although these latter species are perciforms like H. burtoni. As previously described (7), the former sequences are designated type I, and the latter are similar to the so-called type II sequences. Thus, we designated our cDNA sequence as encoding the type I GnRH-R in H. burtoni.
We constructed a phylogenetic tree from the available sequences of expressed GnRH-R genes to examine the pervasiveness of multiple GnRH-R types. It is evident from the GnRH-R phylogenetic tree (Fig. 1C) that the type I and II GnRH-R diverged early in vertebrate evolution and are not recent duplications or necessarily limited to teleosts. Indeed, both teleost and amphibian sequences are divided between two branches of the tree. We have amplified a fragment of a putative type II receptor gene in H. burtoni, as shown in the phylogenetic tree. Based on the phylogenetic relationships (Fig. 1C), goldfish IA and IB receptors (6) appear to be a relatively recent duplication. In contrast, no second type I receptor has been identified in the H. burtoni genomic DNA.
Location of GnRH-R in H. burtoni tissue
The location of GnRH-R could suggest its physiological roles. RT-PCR revealed that the GnRH-R-I mRNA has widespread distribution in the brain (Fig. 2), in contrast to the spatially distinct expression of the three endogenous forms of GnRH in this species (11). GnRH-R-I mRNA is also strongly expressed in testes, kidney, and retina (Fig. 2). The expression of this mRNA in both retina, which contains GnRH3, and pituitary, which contains GnRH1, strongly indicates that this receptor is probably activated by multiple forms of GnRH in vivo.
Fig. 2.
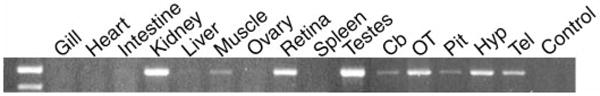
Distribution of GnRH-R in H. burtoni tissue assessed using RT-PCR. Primers based on H. burtoni GnRH-R were used on cDNA isolated from each tissue shown (see Materials and Methods). The first lane contains markers for 500 and 400 bp. Cb, Cerebellum; OT, optic tectum; Pit, pituitary; Hyp, hypothalamus; Tel, telencephalon; Control, RT without RNA, used as the PCR template.
Function of the GnRH-R in H. burtoni
To discover whether the GnRH-R cDNA encoded a functional receptor we analyzed its function in vivo. Various GnRH agonists showed both specific binding to the transfected cells as well as GnRH-stimulated IP production, revealing that the H. burtoni GnRH receptor cDNA encodes a functional, membrane-bound receptor that can respond to all three forms of GnRH expressed in H. burtoni. Clearly, GnRH agonists bind competitively with radiolabeled GnRH to this receptor (Fig. 3A). Furthermore, the receptor does couple to G proteins in COS-1 cells, as evidenced by the ability of GnRH agonists to initiate a second messenger cascade involving inositol phosphate (Fig. 3B).
Fig. 3.
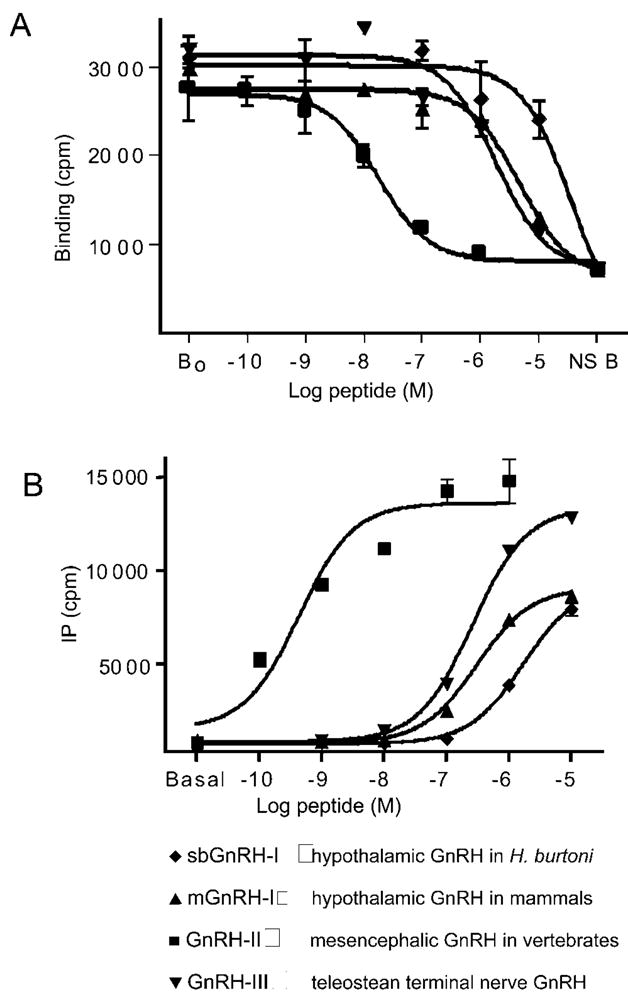
Functional testing of the expressed cDNA for GnRH-R. Illustrated is the binding of GnRH and the production of IP in response to GnRH (see Materials and Methods). A, Whole cell competitive binding of 125I-[His5,D-Tyr6]GnRH after incubation with GnRH isoforms. B, Inositol phosphate in COS-1 cells after stimulation with GnRH.
Some synthetic GnRH agonists showed very high potency, as in other species (6), but in particular, GnRH2 demonstrated much higher binding affinity (Table 2) and potency for IP production (Table 1) than the native GnRH1, mammalian GnRH1 or GnRH3, as has been shown in other nonmammalian species (5, 6, 20, 21). The concordance of this receptor’s characteristics with those of goldfish and catfish (5, 6) is perhaps to be expected from the high sequence homology among them (Fig. 1B).
TABLE 2.
IC50 values for the competitive binding of 125I-[His5,D-Tyr6]GnRH with GnRH agonists in COS-1 cells transiently transfected with the H. burtoni GnRH-R
| Peptide | IC50 (nM) |
|---|---|
| [His5,Trp7,Tyr8]GnRH (GnRH2) | 14.2 ± 5.5 |
| [Trp7,Leu8]GnRH (GnRH3) | 2486.7 ± 888.7 |
| [Arg8]GnRH (mGnRH1) | 2615 ± 2821.4 |
| [Ser8]GnRH (sbGnRH1) | 30233.3 ± 8575.7 |
Data are calculated as the mean of three separate experiments.
It was shown previously that the IP production of GnRH analogs, differing at position 8, reveals functional differences between the goldfish IA and IB GnRH-R subtypes (6). As H. burtoni is not clearly closer to either goldfish GnRH-R subtype (gfIA or gfIB) in sequence identity (Fig. 1B), we used these analogs to examine whether a similarity in function could be found (Fig. 4A). Indeed, whereas gfIB showed reduced IP production for [His8]GnRH vs. other position 8 analogs, gfIA and the H. burtoni GnRH-R lack this characteristic. This prompted an examination of the putative binding regions of the three sequences (Fig. 4B), showing that the amino acid polarities of H. burtoni and gfIA are, unlike gfIB, identical in these regions.
Fig. 4.

A, Comparison of potencies among GnRH analogs that differ at a single residue, position 8. ED50 is plotted for four different GnRH agonists for IP production in COS-1 cells transiently transfected with the H. burtoni GnRH-R or the goldfish GnRH-R subtypes (6). B, Comparison of presumed binding regions of three GnRH-Rs by amino acids and by polarities of amino acids. The first extracellular loop (EC1) and the third extracellular loop (EC3) are compared for H. burtoni and the two goldfish GnRH-R forms.
Discussion
The H. burtoni GnRH receptor conforms to the general structure of other species, a seven-transmembrane domain, G protein-coupled receptor. As in other fish GnRH-Rs, but unlike mammalian GnRH-Rs, there is an intracellular tail at the C-terminus, which is characteristic of G protein-coupled receptors.
The widespread distribution of GnRH-R in both the brain and body confirms and extends data from other teleosts (6, 22, 23). Outside the hypothalamus-pituitary-gonadal axis, the roles of GnRHs and their receptors are largely unknown. For example, the presence of GnRH in the retina and its action on retinal neurons have been known for several years (24–28), although its behavioral significance remains unknown. The source of retinal GnRH is the contralateral terminal nerve (29–31), which is found in all groups of vertebrates (32). The widespread distribution of GnRH-R in mammalian brains (33; reviewed in Ref. 34) has led to speculation that GnRHs play roles beyond reproduction, including vision, emotion, and memory. What exactly those roles might be remains to be discovered.
Like GnRH (10, 35, 36), GnRH-R mRNA is found in many tissues outside the nervous system. In H. burtoni, the kidney and testes show relatively greater abundance of GnRH-R mRNA. The roles of both GnRH (10, 36) and GnRH-R in the kidney are unknown. Also, in rats GnRH-R mRNA is found in the adrenals, but not the kidney (33), whereas in fish the renal system is inseparable from the kidney, suggesting that the current data reflect a role for the renal system rather than the kidneys. Our positive results with testes were predictable, whereas our negative results with ovaries were surprising, given previous mRNA data on mammals and fish (6, 22, 33, 37). However, previous GnRH-R RT-PCR results have suggested lower abundance in fish ovaries compared with testes (22). Accordingly, we were able to demonstrate the presence of GnRH-R mRNA in the ovaries only through reamplification (data not shown). As GnRH-R mRNA is known to be regulated by estrogens (33, 38–41), it is possible that GnRH-R mRNA abundance in the ovary depends on reproductive state, which was not strictly monitored in the present study.
H. burtoni expresses three different GnRH peptides in relatively restricted locations (11), suggesting that the specific forms of GnRH may play several different roles. With three distinct ligands, one might expect a priori that there would be distinct receptor types for each. In fact, evidence for duplicate GnRH-Rs has been found in genomic DNA from several species (7). Likewise, analysis of H. burtoni genomic DNA with degenerate primers yielded a novel sequence with high homology to other type II GnRH-R, in addition to the type I H. burtoni GnRH-R characterized here. To date, however, only tetraploid species [goldfish (6) and Xenopus (Troskie, B., N. Illing, and R. Millar, unpublished data)] have been shown to express more than one GnRH receptor. Evidence against the widespread existence of a second form is that radiolabeled GnRH apparently binds to only one high affinity class of sites in pituitary membranes in African catfish (20, 42) and rats (43–45), as opposed to the two distinguishable binding affinities in goldfish (46). On the other hand, since both type I and type II GnRH-R sequences are expressed in perciform fish, it seems likely that two types of GnRH-R are expressed in individual perciform species, which appear to be diploid (47). Regardless of whether the putative type II gene is expressed, the distribution and binding properties of the H. burtoni type I GnRH-R strongly suggest that all three GnRH forms do act on the type I receptor characterized here. Therefore, it seems unlikely that individual GnRH forms in vivo interact only with specific receptors.
In comparison to the goldfish subtypes IA and IB, the H. burtoni GnRH-R (type I) has a slightly higher similarity to subtype A, but this in itself is insufficient evidence to place it squarely in the subtype A category. Furthermore, there are few differences among these three receptors in terms of relative affinities for GnRH agonists (6). However, the slight differences in sequence and affinity create a natural experiment in transduction efficacy. Whereas goldfish GnRH-R subtype B shows reduced IP production for [His8]GnRH vs. other position 8 analogs, subtype A and the H. burtoni GnRH-R lack this characteristic. Binding affinity, and therefore ligand discrimination and ligand efficacy, have previously been localized to the extracellular ends of the second and seventh helixes (extracellular loops 1 and 3) of GnRH-R (48–50). In particular, although it has become clear that no one amino acid can be held responsible for ligand binding (5, 7), the polarities and relative positions of amino acid side-chains have repeatedly been shown to be crucial for ligand binding. Following this track, despite the variability among these three sequences for EC1 and EC3, the polarities of the amino acid side-chains are very consistent among the three forms, with the only deviations being in goldfish subtype B. Thus, this small difference in affinities may result from a few differences in amino acid polarities.
Acknowledgments
We thank Anna Greenwood and Michael Vagell for insightful comments on the manuscript.
Footnotes
This work was supported by the National Research Foundation (South Africa), the Medical Research Council (South Africa), the University of Cape Town, NIH Grants HD-07493 (to R.B.W.) and NS-34950 (to R.D.F.), and a Jacob Javits Investigator Award (to R.D.F.).
References
- 1.Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart J, Mertz LM, Catt KJ. Molecular cloning and expression of cDNA encoding the murine gonadotropin-releasing hormone receptor. J Biol Chem. 1992;267:21281–21284. [PubMed] [Google Scholar]
- 3.Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–499. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- 4.Troskie BE, Hapgood JP, Millar RP, Illing N. cDNA cloning, gene expression, and ligand selectivity of a novel gonadotropin-releasing hormone receptor expressed in the pituitary and midbrain of Xenopus laevis. Endocrinology. 2000;141:1764–1771. doi: 10.1210/endo.141.5.7453. [DOI] [PubMed] [Google Scholar]
- 5.Tensen C, Okuzawa K, Blomenrohr M, Rebers F, Leurs R, Bogerd J, Schulz R, Goos H. Distinct efficacies for two endogenous ligands on a single cognate gonadoliberin receptor. Eur J Biochem. 1997;243:134–140. doi: 10.1111/j.1432-1033.1997.0134a.x. [DOI] [PubMed] [Google Scholar]
- 6.Illing N, Troskie BE, Nahorniak CS, Hapgood JP, Peter RE, Millar RP. Two gonadotropin-releasing hormone receptor subtypes with distinct ligand selectivity and differential distribution in brain and pituitary in the goldfish (Carassius auratus) Proc Natl Acad Sci USA. 1999;96:2526–2531. doi: 10.1073/pnas.96.5.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troskie B, Illing N, Rumbak E, Sun YM, Hapgood J, Sealfon S, Conklin D, Millar R. Identification of three putative GnRH receptor subtypes in vertebrates. Gen Comp Endocrinol. 1998;112:296–302. doi: 10.1006/gcen.1998.7156. [DOI] [PubMed] [Google Scholar]
- 8.Millar R, Conklin D, Lofton-Day C, Hutchinson E, Troskie B, Illing N, Sealfon SC, Hapgood J. A novel human GnRH receptor homolog gene: abundant and wide tissue distribution of the antisense transcript. J Endocrinol. 1999;162:117–126. doi: 10.1677/joe.0.1620117. [DOI] [PubMed] [Google Scholar]
- 9.Francis RC, Soma K, Fernald RD. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci USA. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White RB, Fernald RD. Genomic structure and expression sites of three gonadotropin-releasing hormone genes in one species. Gen Comp Endocrinol. 1998;112:17–25. doi: 10.1006/gcen.1998.7125. [DOI] [PubMed] [Google Scholar]
- 11.White SA, Kasten TL, Bond CT, Adelman JP, Fernald RD. Three gonadotropin-releasing hormone genes in one organism suggest novel roles for an ancient peptide. Proc Natl Acad Sci USA. 1995;92:8363–8367. doi: 10.1073/pnas.92.18.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan CA, Fromme BJ, Davidson JS, Millar RP. A high affinity gonadotropin-releasing hormone (GnRH) tracer, radioiodinated at position 6, facilitates analysis of mutant GnRH receptors. Endocrinology. 1998;139:4115–4119. doi: 10.1210/endo.139.10.6260. [DOI] [PubMed] [Google Scholar]
- 13.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) 4. Sinauer Associates; Sunderland: 2000. [Google Scholar]
- 14.King JA, Fidler A, Lawrence S, Adam T, Millar RP, Katz A. Cloning and expression, pharmacological characterization, and internalization kinetics of the pituitary GnRH receptor in a metatherian species of mammal. Gen Comp Endocrinol. 2000;117:439–448. doi: 10.1006/gcen.1999.7418. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Sealfon SC. Structure of the mouse gonadotropin-releasing hormone receptor gene: variant transcripts generated by alternative processing. DNA Cell Biol. 1994;13:605–614. doi: 10.1089/dna.1994.13.605. [DOI] [PubMed] [Google Scholar]
- 16.Campion CE, Turzillo AM, Clay CM. The gene encoding the ovine gonadotropin-releasing hormone (GnRH) receptor: cloning and initial characterization. Gene. 1996;170:277–280. doi: 10.1016/0378-1119(96)00042-x. [DOI] [PubMed] [Google Scholar]
- 17.Fan NC, Jeung EB, Peng C, Olofsson JI, Krisinger J, Leung PC. The human gonadotropin-releasing hormone (GnRH) receptor gene: cloning, genomic organization and chromosomal assignment. Mol Cell Endocrinol. 1994;103:R1–R6. doi: 10.1016/0303-7207(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 18.Hauser F, Sondergaard L, Grimmelikhuijzen CJ. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem Biophys Res Commun. 1998;249:822–828. doi: 10.1006/bbrc.1998.9230. [DOI] [PubMed] [Google Scholar]
- 19.Reinhart J, Xiao S, Arora KK, Catt KJ. Structural organization and characterization of the promoter region of the rat gonadotropin-releasing hormone receptor gene. Mol Cell Endocrinol. 1997;130:1–12. doi: 10.1016/s0303-7207(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 20.Schulz RW, Bosma PT, Zandbergen MA, Van der Sanden MC, Van Dijk W, Peute J, Bogerd J, Goos HJ. Two gonadotropin-releasing hormones in the African catfish, Clarias gariepinus: localization, pituitary receptor binding, and gonadotropin release activity. Endocrinology. 1993;133:1569–1577. doi: 10.1210/endo.133.4.8404596. [DOI] [PubMed] [Google Scholar]
- 21.Troskie B, King JA, Millar RP, Peng YY, Kim J, Figueras H, Illing N. Chicken GnRH II-like peptides and a GnRH receptor selective for chicken GnRH II in amphibian sympathetic ganglia. Neuroendocrinology. 1997;65:396–402. doi: 10.1159/000127202. [DOI] [PubMed] [Google Scholar]
- 22.Yu K-L, He M-L, Chik C-C, Lin X-W, Chang JP, Peter RE. mRNA expression of gonadotropin-releasing hormones (GnRHs) and GnRH receptor in goldfish. General and Comparative Endocrinology. 1998;112:303–311. doi: 10.1006/gcen.1998.7137. [DOI] [PubMed] [Google Scholar]
- 23.Okubo K, Suetake H, Usami T, Aida K. Molecular cloning and tissue-specific expression of a gonadotropin-releasing hormone receptor in the Japanese eel. Gen Comp Endocrinol. 2000;119:181–192. doi: 10.1006/gcen.2000.7511. [DOI] [PubMed] [Google Scholar]
- 24.Stell WK, Walker SE, Chohan KS, Ball AK. The goldfish nervus terminalis: a luteinizing-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc Natl Acad Sci USA. 1984;81:940–944. doi: 10.1073/pnas.81.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtsuka T, Kawamata K, Stell WK. Immunocytochemical studies of centrifugal fiber in the goldfish retina. 11th Taniguchi International Symposium on Visual Science, S141–S150; Katata, Japan. 1989. [DOI] [PubMed] [Google Scholar]
- 26.Zucker CL, Dowling JE. Centrifugal fibers synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]
- 27.Walker SE, Stell WK. Gonadotropin-releasing hormone (GnRF), molluscan cardioexcitatory peptide (FMRFamide), enkephalin and related neuropeptides affect goldfish retinal ganglion cell activity. Brain Res. 1986;384:262–273. doi: 10.1016/0006-8993(86)91162-5. [DOI] [PubMed] [Google Scholar]
- 28.Umino O, Dowling JE. Dopamine release from interplexiform cells in the retina: effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J Neurosci. 1991;11:3034–3046. doi: 10.1523/JNEUROSCI.11-10-03034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Münz H, Claas B, Stumpf WE, Jennes L. Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LHRH)-immunoreactive telencephalic neurons in teleostean fishes. Cell Tissue Res. 1982;222:313–323. doi: 10.1007/BF00213215. [DOI] [PubMed] [Google Scholar]
- 30.Crapon de Caprona M, Fritzsch B. The development of the retinopetal nucleus olfacto-retinalis of two cichlid fish as revealed by horseradish peroxidase. Dev Brain Res. 1983;11:281–301. doi: 10.1016/0165-3806(83)90227-4. [DOI] [PubMed] [Google Scholar]
- 31.Wilm C, Fritzsch B. Ipsilateral retinopetal projection of the nucleus olfactoretinalis (NOR) during development and regeneration: a DiI study in a cichlid fish. J Neurobiol. 1993;24:70–79. doi: 10.1002/neu.480240106. [DOI] [PubMed] [Google Scholar]
- 32.Demski LS. Terminal nerve complex. Acta Anat. 1993;148:81–95. doi: 10.1159/000147528. [DOI] [PubMed] [Google Scholar]
- 33.Kakar SS, Grantham K, Musgrove LC, Devor D, Sellers JC, Neill JD. Rat gonadotropin-releasing hormone (GnRH) receptor: tissue expression and hormonal regulation of its mRNA. Mol Cell Endocrinol. 1994;101:151–157. doi: 10.1016/0303-7207(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 34.Jennes L, Eyigor O, Janovick JA, Conn PM. Brain gonadotropin releasing hormone receptors: localization and regulation. Recent Prog Horm Res. 1997;52:475–490. [PubMed] [Google Scholar]
- 35.Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98:57–62. [PubMed] [Google Scholar]
- 36.White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci USA. 1998;95:305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahk JY, Hyun JS, Chung SH, Lee H, Kim MO, Lee BH, Choi WS. Stage specific identification of the expression of GnRH mRNA and localization of the GnRH receptor in mature rat and adult human testis. J Urology. 1995;154:1958–1961. [PubMed] [Google Scholar]
- 38.Bauer-Dantoin AC, Hollenberg AN, Jameson JL. Dynamic regulation of gonadotropin-releasing hormone receptor mRNA levels in the anterior pituitary gland during the rat estrous cycle. Endocrinology. 1993;133:1911–1914. doi: 10.1210/endo.133.4.8404635. [DOI] [PubMed] [Google Scholar]
- 39.Wu JC, Sealfon SC, Miller WL. Gonadal hormones and gonadotropin-releasing hormone (GnRH) alter messenger ribonucleic acid levels for GnRH receptors in sheep. Endocrinology. 1994;134:1846–1850. doi: 10.1210/endo.134.4.8137751. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 41.Cowley MA, Rao A, Wright PJ, Illing N, Millar RP, Clarke IJ. Evidence for differential regulation of multiple transcripts of the gonadotropin releasing hormone receptor in the ovine pituitary gland; effect of estrogen. Mol Cell Endocrinol. 1998;146:141–149. doi: 10.1016/s0303-7207(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 42.De Leeuw R, Conn PM, Veer CVt, Goos HJT, Van Oordt PGWJ. Characterization of the receptor for gonadotropin-releasing hormone in the pituitary of the African catfish, Clarias gariepinus. Fish Physiol Biochem. 1988;5:99–107. doi: 10.1007/BF01875646. [DOI] [PubMed] [Google Scholar]
- 43.Savoy-Moore RT, Schwartz NB, Duncan JA, Marshall JC. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science. 1980;209:942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- 44.Frager MS, Pieper DR, Tonetta SA, Duncan JA, Marshall JC. Pituitary gonadotropin-releasing hormone receptors. Effects of castration, steroid replacement, and the role of gonadotropin-releasing hormone in modulating receptors in the rat. J Clin Invest. 1981;67:615–623. doi: 10.1172/JCI110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayton RN, Solano AR, Garcia-Vela A, Dufau ML, Catt KJ. Regulation of pituitary receptors for gonadotropin-releasing hormone during the rat estrous cycle. Endocrinology. 1980;107:699–706. doi: 10.1210/endo-107-3-699. [DOI] [PubMed] [Google Scholar]
- 46.Habibi HR, Peter RE, Sokolowska M, Rivier JE, Vale WW. Characterization of gonadotropin-releasing hormone (GnRH) binding to pituitary receptors in goldfish (Carassius auratus) Biol Reprod. 1987;36:844–853. doi: 10.1095/biolreprod36.4.844. [DOI] [PubMed] [Google Scholar]
- 47.Thompson KW. Karyotypes of 6 species of African Cichlidae (Pisces: Perciformes) Experientia. 1981;37:351–352. doi: 10.1007/BF01959857. [DOI] [PubMed] [Google Scholar]
- 48.Flanagan CA, Becker II, Davidson JS, Wakefield IK, Zhou W, Sealfon SC, Millar RP. Glutamate 301 of the mouse gonadotropin-releasing hormone receptor confers specificity for arginine 8 of mammalian gonadotropin-releasing hormone. J Biol Chem. 1994;269:22636–22641. [PubMed] [Google Scholar]
- 49.Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, Millar RP, Sealfon SC. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol. 1994;45:165–170. [PubMed] [Google Scholar]
- 50.Davidson JS, McArdle CA, Davies P, Elario R, Flanagan CA, Millar RP. Asn-102 of the gonadotropin-releasing hormone receptor is a critical determinant of potency for agonists containing C-terminal glycinamide. J Biol Chem. 1996;271:15510–15514. doi: 10.1074/jbc.271.26.15510. [DOI] [PubMed] [Google Scholar]