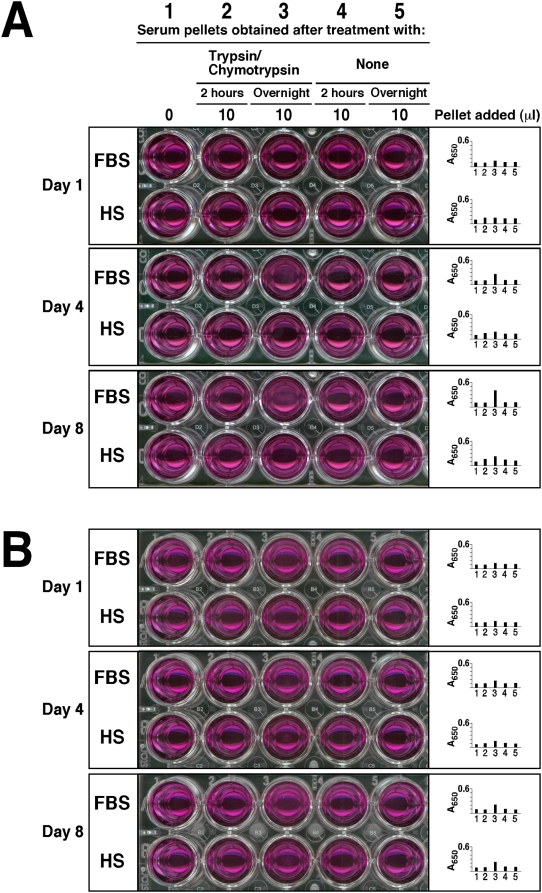
Figure 12. Trypsin and chymotrypsin treatment of FBS and HS generates pellets that have particle-seeding activity when inoculated into serum-free medium.
Seeding-pellets were prepared by adding trypsin (A) or chymotrypsin (B) at 0.5% to either FBS or HS, followed by incubation at 37°C for 2 hours or overnight, then centrifugation and washing as described in the Materials and Methods . 10 µl aliquots of these resuspended pellets (wells 2 and 3, with well 2 corresponding to 2 hours of serum incubation after protease treatment and well 3 corresponding to overnight incubation of the same protease treatment) were then inoculated into 1 ml of DMEM and incubated in cell culture conditions for the time indicated on the left. “Day 1” refers to the day of pellet inoculation. As control, sera that received no addition of proteases were also incubated, centrifuged, and washed the same way, after which the pellets (barely visible in most control specimens) were inoculated into DMEM and incubated exactly as before (wells 4 and 5, described as “None”). As an additional control, DMEM alone was incubated without any inoculation (well 1). Note the time-dependent increase in turbidity associated with either protease treatment, more noticeable with the pellets that had been obtained from sera treated with proteases overnight as contrasted with 2 hour treatment (compare between visuals obtained for wells 3 and 2). Turbidity was usually higher with the FBS pellets compared to HS pellets. Note also the different amounts of turbidity seen with trypsin (A) vs. chymotrypsin (B) treatments; turbidities were generally higher with trypsin treatment. Control wells inoculated with pellets obtained from serum that received no protease treatment (wells 4 and 5), or containing DMEM alone (well 1), produced either little or no visible turbidity.