FIG. 3.
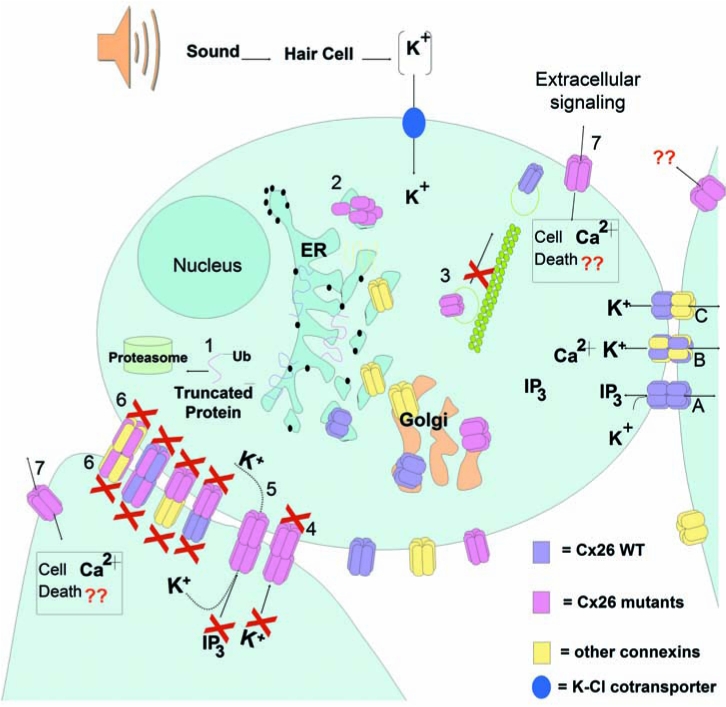
Pathogenic mechanism of deafness-associated Cx26 mutations. Wild-type connexins oligomerize in the ER/Golgi. Hemichannels traffic to plasma membrane through the secretory pathway by a cytoskeletal-dependent mechanism. Epithelial and supporting cells in the cochlea express both Cx26 and Cx30. (A) Cx26 homomeric GJCh are permeable to ions, like K+, and bigger molecules, like IP3. Cx30 homomeric GJCh have high permeability to K+ but lower permeability to IP3. (B) Heteromeric Cx26–Cx30 GJCh. (C) Heterotypic channels. Deafness-associated Cx26 mutations may produce 1. Truncated protein connexin subunits; 2. Oligomerization defects impeding the assembly of hemichannels; 3. Defective trafficking of the hemichannels, impeding targeting to the plasma membrana; 4. Nonfunctional channels; normal trafficking and assembly into the plasma membrane and gap-junction plaque formation, but the GJCh are closed or their pore structure severely affected, impeding the diffusion of ions and small metabolites; 5. Functional channels permeable to ions but with reduced permeability to bigger molecules like IP3, affecting propagation of calcium waves or other metabolites; 6. Mutant Cx26 that can act as dominant negative of co-expressed wild-type connexins. Mutant Cx26 can oligomerize with wild-type connexins, producing nonfunctional heteromeric channels. Heterotypic combination between mutant Cx26 hemichannel and wild-type hemichannels can also lead to nonfunctional channels; 7. Aberrant functionality of free hemichannels in the plasma membrane, allowing an increase in plasma-membrane permeability that may lead to cell death due to either loss of important intracellular metabolites (like ATP or NAD), or increase intracellular calcium concentration. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
