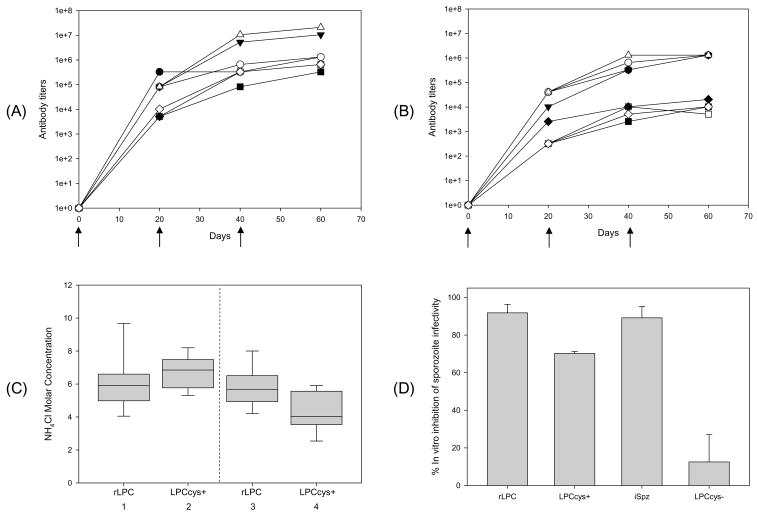
Figure 3.
Kinetics of antibody response, anti-LPC affinities and functional activity of antibodies elicited by immunization of mice with rLPC or synthetic LPCcys+. Antibody titers were measured 20 days after each immunization of CAF1/J (A) or BALB/c mice (B) with rLPC or synthetic LPCcys+ and expressed as reciprocal geometric mean antibody titers in pooled sera. The data are representative of one out of two independent experiments. Anti-sera from mice immunized with rLPC (closed symbols) or synthetic LPCcys+ (open symbols) were tested by ELISA with rLPC (●○), synthetic LPCcys+ (▼;△), synthetic peptide representing the B-cell epitope (□■) or synthetic peptide representing the T cell epitope (◇◆). (C) Antibody avidity (expressed as concentration of ammonium thiocyanate required to dissociate 50% of bound peptide) measured 20 days after the third immunization of CAF1/J (lanes 1 and 2) or BALB/c mice (lanes 3 and 4). Avidity of anti-rLPC antibody is shown in lanes 1 (CAF1/J mice) and 3 (BALB/c); anti-synthetic LPCcys+ in lanes 2 (CAF1/J) and 4 (BALB/c). The box-whisker plot represents median, 25th and 75th percentiles from six mice. (D) Sporozoite-neutralizing antibodies \in sera of BALB/c mice immunized with rLPC or synthetic LPCcys+. Viable P. yoelii sporozoites \were incubated for 40 minutes on ice with pools of immune sera, from BALB/c mice immunized with rLPC, LPCcys+, LPCcys− or irradiated sporozoites (iSpz), prior to the infection of HepG2 hepatoma cells. P. yoelii parasite loads were determined measuring 18S rRNA using RT-PCR after 40 hour incubation. The results are expressed as percentage of in vitro inhibition of sporozoite infectivity determined as follows: % Inhibition = [(1 − copy number obtained with immune sera/copy number obtained with pre-immune sera) × 100]