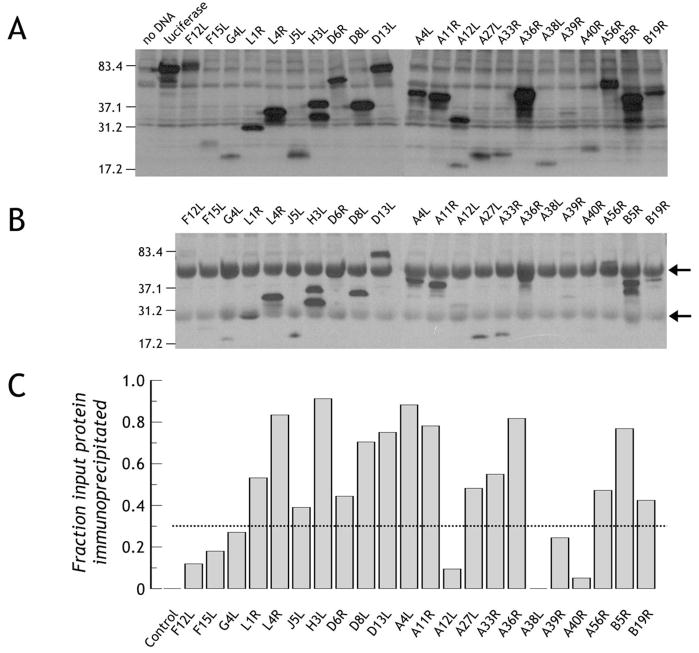
Figure 2. Production of vaccinia proteins and their immunoprecipitation with VIg.
A. Production of biotin-labeled vaccinia protein domains. The desired vaccinia domains were produced incorporating tRNAlys precharged with ε-amino-biotinylated lysine. Synthesized proteins were detected with streptavidin-horse radish peroxidase. B. Immunoprecipitation of synthetically labeled proteins by human anti-vaccinia antibody. Identical amounts of protein to those loaded and detected in panel A were incubated with human anti-vaccinia hyperimmune IgG (VIg) and bound biotinylated protein was immunoprecipitated using Protein G-agarose. Following gel electrophoresis and transfer, bound protein was detected as in panel A. Arrows represent human Ig H and L chains, respectively. C. Broad reactivity of VIg to the synthetic antigen array. For each protein in panel A and panel B, the band relative intensity was normalized to luciferase run on the same gel. Since identical amounts of luciferase were run on each gel, the ratio of immunoprecipitated protein (panel B) to total protein loaded (panel A) gives a broad measure of VIg reactivity to specific antigens. Using a conservative cut-off threshold of 0.35 (indicated by the dotted line, strong reactivity is seen to L1, L4, J5, H3, D6, D8, D13, A4, A11, A27, A33, A36, A56, B5 and B19. Control is luciferase precipitated by VIg.