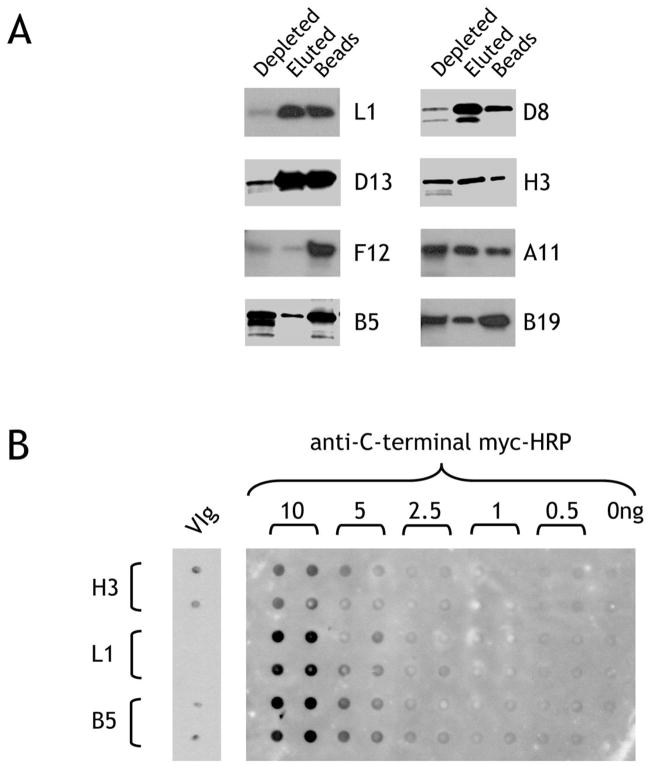
Figure 3. Purification of His(6)-tagged proteins and reactivity on nitrocellulose.
A. Purification of vaccinia proteins synthesized in vitro. Reticulocyte lysate containing the newly-synthesized proteins were incubated with MagZ magnetic beads to bind the His(6)-tagged proteins. After washing and elution, the depleted lysate, eluate, or beads were run on a reducing SDS-PAGE gel and biotinylated protein detected by binding of streptavidin-peroxidase. B. Immobilization on nitrocellulose may affect the presentation of antigenic epitopes. Recombinant biotin-labeled H3, L1 and B5 vaccinia proteins purified by His-tag affinity were spotted in quadruplicate on nitrocellulose and probed with anti-myc-HRP directed against the C-terminal myc tag followed by chemiluminescent assay (right-hand panel). The same proteins were blotted in duplicate (10ng/spot) on a separate blot, incubated with VIg and detected with anti-human IgG-HRP.