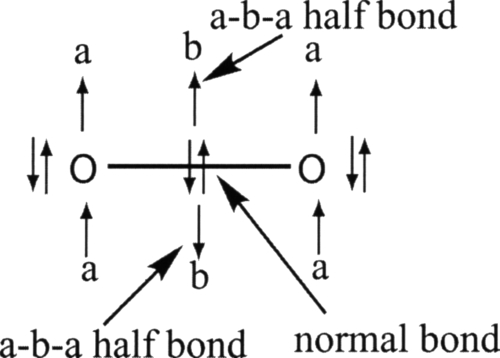
Figure 1.
Electronic structure displaying two a-b-a half bonds. This representation points out the fact that each oxygen center bears some radical character and that the total bond order of the molecule is 2 (1+2⋆1∕2). Each a-b-a half bond actually displays only one unpaired electron and not three since the beta electron can actually be paired with one of the alpha electon localized on the oxygen. This explains why the system of three electrons is described altogether as an a-b-a half bond. Extended NBO analysis and a sligthly different representation of dioxygen molecules is given in Ref. 66.