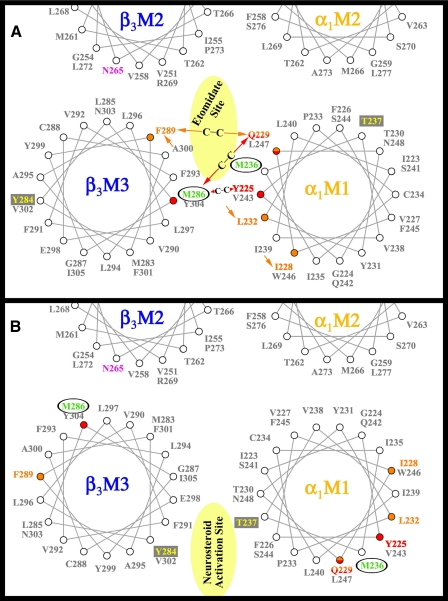
FIGURE 3.
Helical wheel representations of the GABAAR β-α interface in the transmembrane domain illustrating the proposed binding sites for etomidate and neurosteroids. The model in A illustrates the orientation of residues from a homology model built on the nicotinic acetylcholine receptor cryo-EM derived structure (PDB: 2BG9) (12), with the residues in αM1 and βM3 photolabeled by [3H]azietomidate (circled residues in green) contributing to a common binding pocket at the β-α subunit interface. Also included in pink is the position in βM2 that functions as a determinant of etomidate/azietomidate anesthetic potency in vivo (7, 29), the residues in αM1 and βM3 identified as sensitivity determinants for direct activation by neurosteroids (boxed residues in yellow) (16), and the positions in αM1 and βM3 that when mutated to Cys can form intersubunit cross-links (red and orange) (22). B illustrates the locations of those residues in an alternative GABAAR model (16).