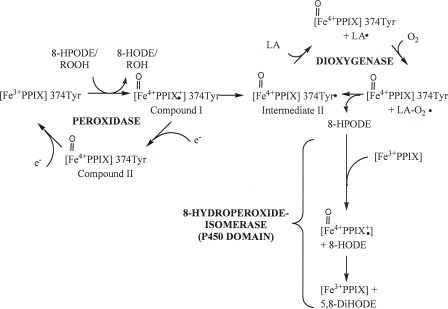
FIGURE 10.
Hypothetical catalytic mechanism of PpoA with linoleic acid as a substrate. The initiating step of catalysis is the reduction of hydroperoxides to the corresponding hydroxides with the formation of compound I. This species is either converted by an intermolecular reduction to intermediate II with the formation of the Tyr374 radical or alternatively reduced by an exogenous electron donor (e.g. TMPD) to compound II and further reduction back to heme, yielding the resting enzyme. The Tyr374 radical is then used for the generation of the linoleoyl radical, and subsequently, molecular oxygen is inserted, resulting in peroxylinoleate. The latter is converted to 8-HPODE by abstracting a hydrogen from Tyr374. Either 8-HPODE serves as the substrate for the 8-hydroperoxide isomerase reaction within the P450 heme thiolate domain in which it is isomerized via the intermediate formation of compound I to 5,8-DiHODE, or alternatively, it serves as a substrate for the peroxidase reaction, yielding 8-HODE. LA, linoleic acid; PPIX, ferric iron protoporphyrin IX.