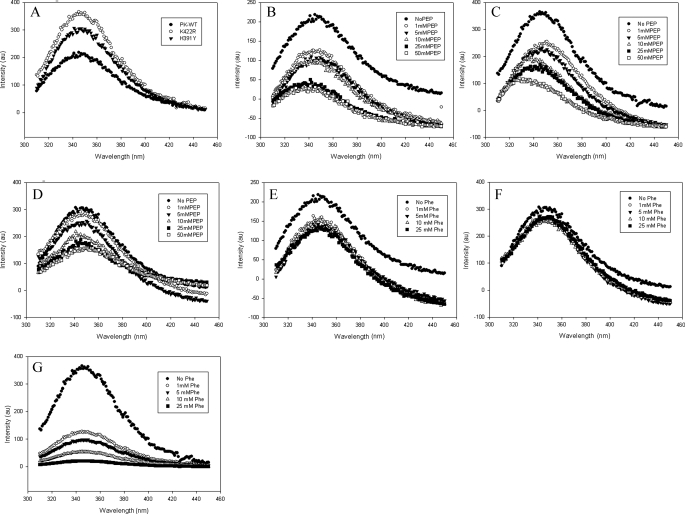
FIGURE 6.
The intrinsic tryptophan fluorescence of the proteins (50 μg/ml) was measured upon excitation at 295 nm and by scanning emission in the range of 310–450 nm. A, emission spectra of PK-WT and the K422R and H391Y mutant proteins. B–D, P-enolpyruvate titration of PK-WT, K422R, and H391Y, respectively, showing quenching with increasing concentrations of P-enolpyruvate (PEP). E–G, PK-WT, H391Y, and K422R, respectively, showing quenching with increasing concentrations of the inhibitor phenylalanine. au, absorbance units.