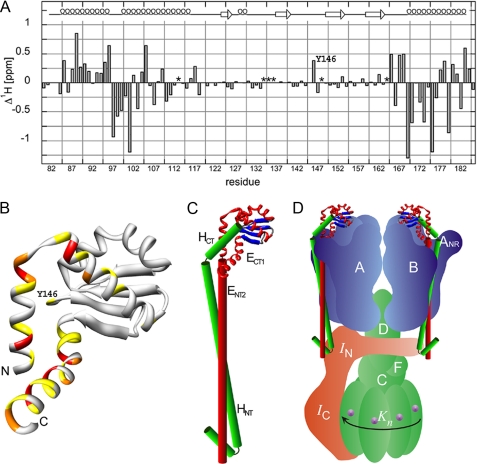
FIGURE 6.
Analysis of ECT1 chemical shift change upon the addition of HCT and a model of the proposed domain arrangement in the full-length EH peripheral stalk complex. Assignment of the 15N HSQC spectra presented in Fig. 4 permitted the calculation of ECT1 amide-proton chemical shift changes (Δ1Hδ) upon binding HCT. A, as can be seen, the majority of residues experiencing the most significant chemical shift perturbation upon HCT binding are located in the N- and C-terminal helices of ECT1 (residues for which no assignments could be obtained in either the ECT1 (Ser113, Gly148, and Ile164) or ECT1HCT (Ser134, Asp135, and Gly136) spectra are marked with an asterisk). B, the positions analogous to the Ta_ECT1 residues undergoing the largest chemical shift changes are highlighted in the available crystal structure of Ph_ECT with one dimer partner removed (Protein Data Bank code 2dma; Ref. 11). The largest change in chemical shift in the middle portion of the structure is experienced by Tyr146, which can be seen located close to the N- and C-terminal α-helices of Ph_ECT in the crystal structure (Ta_E Y146 corresponds to Ph_E M157). C, HCT, modeled as an α-helix and guided by the data presented here, is docked onto the Ph_ECT crystal structure monomer. Continuing below the globular ECT1HCT domain, the N-terminal domain, ENT2HNT, is modeled as a pair of parallel helices, representing the coiled-coil domain. We speculate that the N-terminal α-helix of HNT is folded back to interact with and stabilize the N-terminal ends of the coiled-coil domain. D, the resulting EH model placed into a schematic model of the T. acidophilum A-ATPase. The stoichiometry of the EH peripheral stalks has recently been determined for the related A/V-ATPase from T. thermophilus (6).