FIGURE 5.
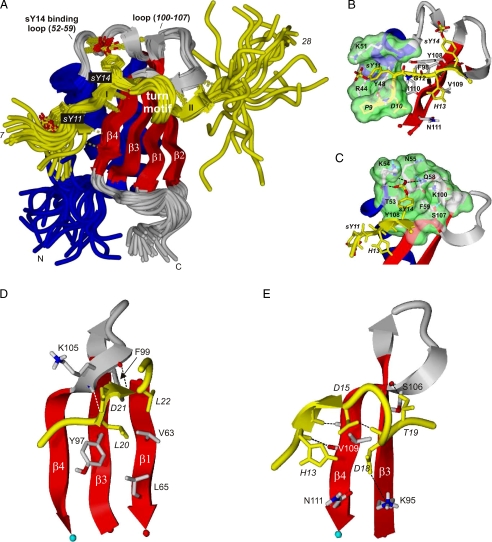
Structure of the CHIPS31-121:C5aR7-28S2 complex. Specific residues belonging to C5aR7-28S2 are annotated by italics. A, overlay of the 25 selected low-energy NMR structures determined of the complex between CHIPS31-121 and C5aR7-28S2. Newly formed β-strands in the peptide are labeled I and II. B, binding pocket of sY11. Residues lining the pocket are labeled. C, binding pocket of sY14. D, view of the binding strand II and the hydrophobic interactions of peptide residues Leu-20 and Leu-22, positioned toward the β-sheet surface of CHIPS. E, view of the binding strand I and turn-motif of the complex. Residues Asp-16 and Lys-17 of the peptide stick out into solution and are omitted from the plot for clarity. Intermolecular interactions are indicated by broken lines. Coloring scheme: C5aR7-28S2 in yellow; the side chains of sY11 and sY14 in yellow sticks and the sulfate oxygens in red; the CHIPS31-121 β-strands in red; the CHIPS31-121 binding loops, the β1-β2 loop, and the C terminus (113-121) in gray; the CHIPS31-121 α-helix and remaining backbone in blue. The solvent-accessible surface surrounding the sulfated tyrosine is colored green.