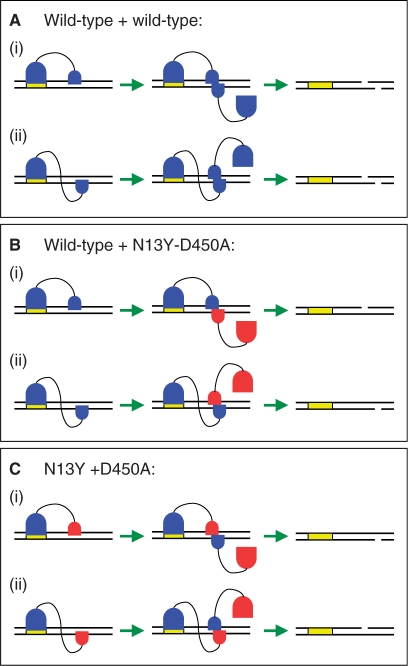
Figure 1.
Experimental strategy. In all three panels, both strands of the DNA duplex are shown, with gaps to indicate cleaved products: the yellow box marks the recognition site for FokI. The FokI monomer is shown as two domains connected by a flexible linker: a (large) DNA recognition domain for specific binding and a (small) catalytic domain for dimerization and DNA cleavage. Functional domains are in blue. Domains inactivated by mutation for either DNA binding (in the large domain) or for catalysis (in the small domain) are in red. The 1° monomer bound to the recognition site could in principle use its catalytic domain to engage the scissile bond in the top strand, leaving the 2° monomer to attack the bottom strand (pathway i in all three panels). Alternatively (pathway ii), the 1° monomer attacks the scissile bond in the bottom strand, in which case the 2° monomer cuts the top strand. (A) With two monomers of wt FokI, both pathways (i) and (ii) lead to the cutting of both strands. (B) With a mixture of wt FokI and the N13Y-D450A double mutant, only the wt enzyme can act as the 1° monomer, while either the wt or the double mutant might act as the 2° monomer. Hence, the double mutant ought to inhibit the reaction on the strand cut by the 2° monomer: the bottom strand in pathway (i); the top in pathway (ii). (C) With the D450A and the N13Y mutants, D450A can bind to the specific site but N13Y cannot. As only N13Y is active, D450A and N13Y have to function as 1° and 2° monomers, respectively so this mixture ought to cut only one strand: the bottom strand in pathway (i); the top in (ii).