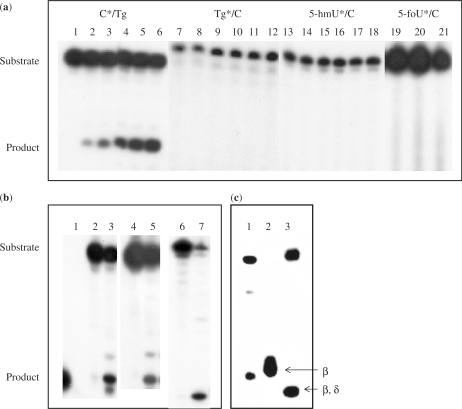
Figure 5.
Cleavage of oligonucleotides containing Tg/C and 5-foU/C mispairs by purified KsgA. (a) The oligonucleotides containing 50 fmol C*/Tg (lanes 1–6), Tg*/C (lanes 7–12), 5-hmU*/C (lanes 13–18) and 5-foU*/C (lanes 19–21) (asterisks indicate labeled strand) were incubated at 37°C for 90 min with increasing amounts of purified KsgA. KsgA was added at 0 (lanes 1, 7, 13 and 19), 12.5 (lanes 2, 8 and 14), 25 (lanes 3, 9 and 15), 50 (lanes 4, 10,16 and 20), 75 (lanes 5, 11, and 17) and 100 (lanes 6, 12, 18 and 21) pmol. (b) Unlabeled 5-foU, Tg or 5-hmU-containing oligonucleotides were annealed to a 32P-labeled oligonucleotide containing C opposite the oxidative modifications of thymine. The double-stranded oligonucleotides (50 fmol) were incubated at 37°C for 60 min without (lanes 2, 4 and 6) or with (lanes 3, 5 and 7) purified KsgA protein (100 pmol). Lane 1, 8-mer marker. (c) 5′-P32-labeled GCCGCCCGCGCGCGCCG/3′-CGGCGGGCTgCGCGCGGC (Tg/C#) (10 fmol) was incubated at 37°C for 60 min with purified GST-KsgA (107 pmol), and 5′-P32-labeled GCCGCCCGTgGCGCGCCG/3′-CGGCGGGCACGCGCGGC (Tg/A#) (10 fmol) with Nth (4 pmol) or Nei (6.5 pmol). The products were separated by denaturing 20% PAGE in gels containing urea. Lane 1, purified GST-KsgA; lane 2, purified GST-Nth (β-elimination), lane 3, purified Nei-his (β,δ-elimination).