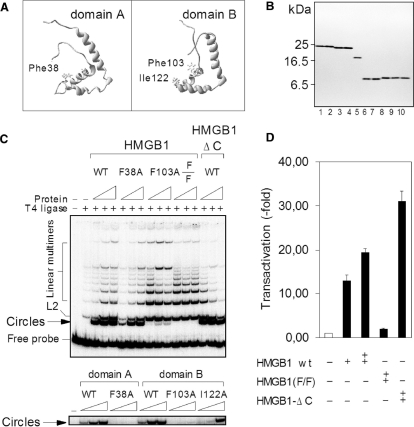
Figure 3.
HMGB1 incapable of DNA bending cannot up-regulate activity of human the topo IIα gene promoter. (A) Position of intercalating residues within the HMGB1 domains A (Phe38) and B (Phe103 and Ile122). (B) Purity of HMGB1, domains A and B, and mutants as revealed by electrophoresis on an SDS/18% polyacrylamide gel and Coomassie blue R-250 staining. Lane 1, wild-type HMGB1; lane 2, HMGB1(F38A); lane 3, HMGB1(F103A); lane 4, HMGB1(F38A/F103A) [designated as F/F in panels (C) and (D)]; lane 5, HMGB1 lacking the C-tail (HMGB1-ΔC or A+B di-domain); lane 6, the wild-type domain A; lane 7, domain A (F38A); lane 8, the wild-type domain B; lane 9, domain B (F103A); lane 10, domain B (F103A/I122A). (C) Effect of mutated intercalating amino acids of HMGB1 or HMGB1 domains A and B on T4 DNA ligase-mediated DNA cyclization. Ligase-mediated circularization assay was carried out with a 32P-labeled 123-bp DNA duplex, and HMGB1 or HMGB1-ΔC at concentrations of 0.05, 1 and 1.5 μM (left to right, upper panel). For experiments with individual HMGB1 domains, the concentrations of the domain A or mutants were 1.5, 3 and 6 μM, and the concentrations of the domain B or mutants were 0.5, 1 and 1.5 μM (left to right, lower panel). The DNA–protein complexes were ligated with T4 DNA ligase and the deproteinised DNA samples were then separated on 5% non-denaturing polyacrylamide gels. (D) Transactivation of the topo IIα gene promoter by the double-mutant of HMGB1 or HMGB1-ΔC. Plasmids encoding HMGB1 or mutants were co-transformed with the plasmid pTIIα-617 into the Saos-2 cells, and the luciferase activity (transactivation) was measured as in Figure 1.